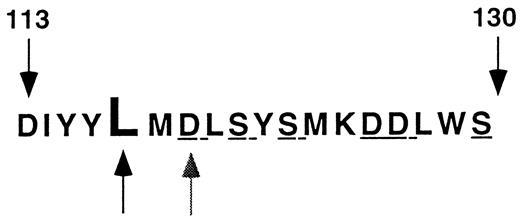Abstract
We report a case of Glanzmann thrombasthenia in a Pakistani child whose platelets express less than 10% of the normal amount of αIIbβ3 on their surface. Single-stranded conformation polymorphism analysis of the exons of the patient's αIIb and β3 genes showed an abnormality in exon 4 of the β3 gene. Direct sequence analysis showed that the patient was homozygous for a T → G nucleotide substitution in this exon, resulting in the replacement of a highly conserved Leu at position 117 with Trp. Heterologous expression of αIIbβ3 containing the β3 mutation in COS-1 cells confirmed the pathogenicity of the Leu117 → Trp substitution and showed that it resulted in the intracellular retention of malfolded αIIbβ3 heterodimers. Additional site-directed mutagenesis at position 117 indicated that, although the smaller hydrophobic amino acid Val could be substituted for the wild-type Leu, the larger hydrophobic amino acids Trp and Phe or the charged amino acids Asp and Lys were not tolerated. These studies indicate that Leu117 in β3 plays a critical role in attaining the correct folded conformation of αIIbβ3. These studies also suggest that the hydrophobic side chain of Leu117 is likely folded into the interior of β3, where it serves to stabilize internal packing of the protein and determines its overall shape.
PLATELET AGGREGATION is responsible for primary hemostasis and results from the cross-linking of activated platelets by fibrinogen and/or von Willebrand factor bound to the integrin αIIbβ3 (GPIIb-IIIa, CD41CD62).1,2 Consequently, inherited abnormalities in either the quantity or function of αIIbβ3 result in the bleeding disorder Glanzmann thrombasthenia.3,4 More than 30 mutations that result in the thrombasthenic phenotype have been identified in either αIIb or β3.5-21 Although some of these mutations have been complex gene rearrangements, gene deletions, mRNA splicing abnormalities, frameshifts, or missense mutations that prevent αIIb or β3 synthesis,5-12 others have been single amino acid substitutions or small deletion or truncations.13-21 The latter have proven to be particularly instructive in understanding αIIbβ3 biology.
Congenital or site-directed mutations involving the region of αIIb that contains its four putative calcium-binding domains6,18-20,22 result in a distinctive phenotype. These mutations neither destabilize αIIb nor prevent the assembly of αIIbβ3 heterodimers. However, assembled heterodimers containing the αIIb mutations are not recognized by heterodimer-specific monoclonal antibodies (MoAbs) and are retained intracellularly, presumably because they are malfolded. In contrast, congenital or site-directed mutations involving a region of β3 extending from residues 119 to 130 do not impair αIIbβ3 expression, although the more proximal mutations impair fibrinogen binding to αIIbβ3.23
We report here studies of a patient with Glanzmann thrombasthenia whose platelets express little, if any, αIIbβ3 on their surface and who is homozygous for a Leu → Trp substitution at β3 residue 117. Unlike previously described mutations in this region of β3, the phenotype resulting from the Leu117 → Trp mutation is identical to that of mutations involving the αIIb calcium binding region. Because both the β3 Leu117 → Trp and the αIIb mutations prevent recognition of αIIbβ3 by heterodimer-specific MoAbs, it is possible that the affected regions of β3 and αIIb are physically associated in the intact αIIbβ3 heterodimer and form the epitopes recognized by these antibodies. Furthermore, because a notable feature of these antibodies is to inhibit fibrinogen binding to αIIbβ3, it is also possible that the affected regions of β3 and αIIb constitute the αIIbβ3 ligand binding site.
MATERIALS AND METHODS
Case report. Patient MK, a Pakistanian child, presented at 1 day of age with petechiae, purpura, and a platelet count of 181,000/μL. When the petechiae recurred at 4 weeks of age, a workup showed absence of platelet aggregation in response to adenosine diphosphate (ADP), epinephrine, and collagen, but a normal response to ristocetin. Blood samples for the studies described below were obtained from the patient and her parents after informed consent. The studies were approved by the Human Subjects Internal Review Committee at the Children's Hospital of Philadelphia.
Flow cytometry. Flow cytometry was performed using a panel of MoAbs conjugated to florescein isothiocyanate (FITC) as described previously.16 The MoAbs used for these studies were A2A9, an MoAb that interacts with an epitope expressed on the extracellular domain of the intact αIIbβ3 heterodimer24,25; B1B5, an MoAb that recognizes an epitope located on the extracellular portion of the 18 nm tail of αIIb proximal to its transmembrane domain25; SSA6, an MoAb that recognizes an epitope located on the extracellular portion of the 18 nm tail of β3 just proximal to its transmembrane domain25; PAC-1, an MoAb that recognizes an epitope located on the extracellular portion of αIIbβ3 and expressed exclusively by its activated conformation26; and AP-1, an MoAb specific for platelet GPIb and a gift of Dr Thomas Kunicki (Scripps Research Institute, La Jolla, CA).27 Measurements of PAC-1 binding were performed after stimulating platelets with 0.2 μmol/L phorbol myristate acetate for 5 minutes at 25°C.
Identification of the thrombasthenic mutation. Genomic DNA containing all of the exons of the αIIb and β3 genes and 500 bp of DNA immediately upstream of each transcriptional start site was isolated from the patient and from normal controls, as previously described.28 Screening for mutations was performed using single-stranded conformation polymorphism analysis (SSCPA)ρ.29 30 Polymerase chain reaction (PCR) amplification was performed in a 100 μL reaction volume containing 500 ng of genomic DNA, 100 μmol/L dNTPs, 1 μL of 32P-dCTP (10 mCi/mL; Dupont, NEN Research Products, Boston, MA), 200 ng of each primer, and 1 U Taq polymerase. Thirty cycles of PCR amplification were performed as follows: denaturation at 94°C for 15 seconds, annealing at 60°C for 30 seconds, and elongation at 72°C for 40 seconds. Equal quantities of the PCR products were used for SSCPA. The PCR products in 95% formamide were heated for 2 minutes at 95°C and loaded onto a 35 × 42.5 cm, 6% DNA sequencing polyacrylamide gel (IBI/VWR Scientific, Bridgeport, NJ) containing no urea. Electrophoresis was performed at 30 watts and 8°C for 2 hours. The completed gels were dried and examined by autoradiography.
DNA fragments that migrated abnormally in the SSCPA gel were directly sequenced using the fmol DNA Cycle Sequencing Kit (Promega, Madison, WI) in a BIOS Oven III thermal cycler (BIOS Laboratories, New Haven, CT), as previously described.16 20 DNA fragments were also subcloned using the commercial TA Cloning Kit (Invitrogen Co, San Diego, CA) and sequenced using Sp6 and T7 primers and a commercial Sequenase sequencing kit (US Biochemicals, Cleveland, OH).
Heterologous expression of recombinant αIIbβ3. To examine their effect on αIIbβ3 assembly and intracellular transport, identified mutations were introduced into the corresponding wild-type sequence using an overlap PCR technique, as previously described.31 PCR amplification was then performed using VENT polymerase (Promega) to decrease the frequency of PCR-induced mutations.32 The resulting PCR products were digested with the endonucleases BstBI (NEB, Inc, Beverly MA) and Mlu I and subcloned into similarly digested αIIb or β3 cDNA in PUC18 (GIBCO/BRL, Gaithersburg, MD). After sequencing to insure the fidelity of the PCR reaction, the DNA was shuttled into the expression vector pMT2ADA.33 Site-directed mutations in αIIb or β3 were produced using the same mutagenesis strategy.
Recombinant αIIbβ3 was expressed in vitro using COS-1 cells, as previously described.16,20 Briefly, COS-1 cells were cotransfected with cDNAs for αIIb and β3 by the lipofection method (Lipofectin Reagent; GIBCO/BRL).16 Forty-eight hours after transfection, the cells were either metabolically labeled with 35S-methionine (Dupont) at 200 μCi/mL for 60 minutes16 or surface-labeled with biotin using 5 mmol/L N-hydroxysulfosuccinimide (NHS-LC) biotin phosphate-buffered saline (PBS) (Pierce, Rockford, IL) for 30 minutes at room temperature.18 The cells were then extracted with 0.02 mol/L Tris-HCl buffer, pH 7.2, containing 1% Triton X-100 and αIIbβ3 was immunoprecipitated using SSA6 or A2A9. To identify proteins on the COS-1 cells surface, biotinylated immunoprecipitated proteins were electrophoresed on a 7% sodium dodecyl sulfate-polyacrylamide gel and transferred to an Immobilon membrane (Millipore, Bedford, MA). The resulting blot was blocked with bovine serum albumin and Tween-20 (Sigma, St Louis, MO) and incubated with 1:4,000 dilution of streptavidin-horseradish peroxidase (Amersham, Arlington Heights, IL) for 1 hour at room temperature. Biotinylated proteins were then identified using the ECL kit (Amersham) according to the manufacturer's instructions.
RESULTS
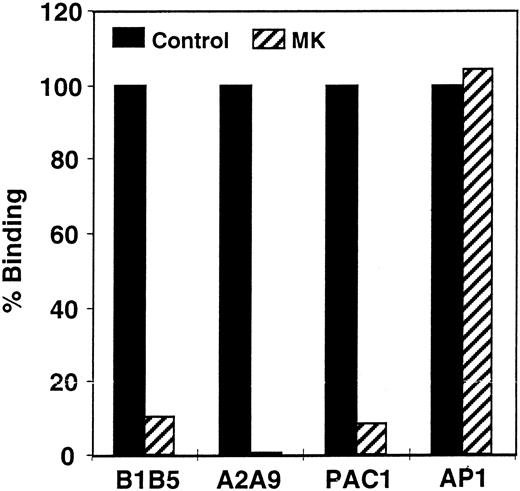
Quantitation of αIIbβ3 on propositus platelets. The inability of platelets from patient MK to aggregate in response to ADP, epinephrine, or collagen suggested that she suffered from Glanzmann thrombasthenia. To confirm this diagnosis, we measured the level of αIIbβ3 on the surface of her platelets by flow cytometry. As shown in Fig 1, when compared with platelets from a normal control, MK's platelets expressed less than 10% of the normal amount of αIIb-β3 when they were stained with the αIIb-specific MoAb B1B5 and the αIIbβ3-specific MoAb A2A9, and there was little or no staining with the activation-dependent, αIIbβ3-specific MoAb PAC-1. In contrast, MK's platelets bound a normal amount of the anti-GPIb MoAb AP-1, confirming that the defect in the patient's platelets was confined to αIIbβ3.
Measurement of the αIIbβ3 content of patient platelets using flow cytometry. Comparison of αIIb-β3 expression on the surface of patient and control platelets using the αIIb-specific MoAb B1B5, the αIIbβ3 heterodimer-specific MoAb A2A9, the activation-dependent anti-αIIbβ3 MoAb PAC-1, and the GP1b-complex specific MoAb AP-1. The data in the figure are expressed as the percentage of antibody binding to patient versus control platelets. Measurements of PAC-1 binding were made after stimulating platelets with 0.2 μmol/L phorbol myristate acetate. (▪) Control; (▨) patient.
Measurement of the αIIbβ3 content of patient platelets using flow cytometry. Comparison of αIIb-β3 expression on the surface of patient and control platelets using the αIIb-specific MoAb B1B5, the αIIbβ3 heterodimer-specific MoAb A2A9, the activation-dependent anti-αIIbβ3 MoAb PAC-1, and the GP1b-complex specific MoAb AP-1. The data in the figure are expressed as the percentage of antibody binding to patient versus control platelets. Measurements of PAC-1 binding were made after stimulating platelets with 0.2 μmol/L phorbol myristate acetate. (▪) Control; (▨) patient.
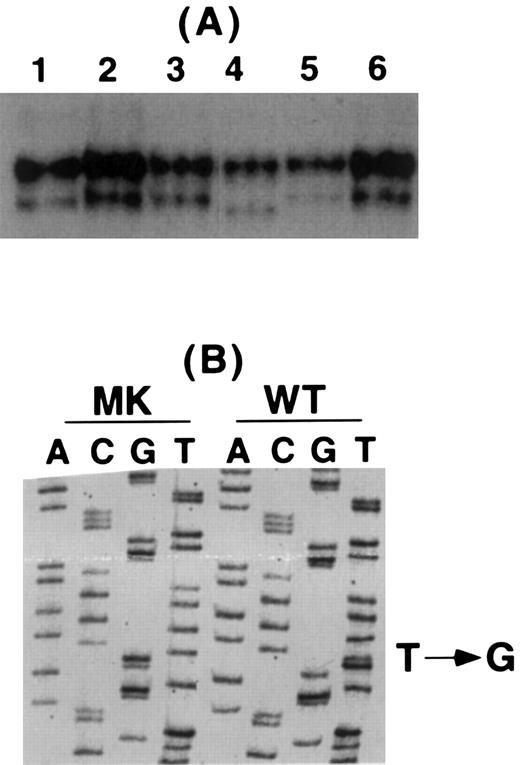
Identification of mutations responsible for the patient's thrombasthenia. To identify the mutation or mutations responsible for the patient's thrombasthenia, genomic DNA from the patient was screened using SSCPA and oligonucleotide primers designed to synthesize DNA fragments containing portions of each exon of the αIIb and β3 genes and the 500 bp immediately upstream of each gene's transcriptional start site.34-36 As shown in Fig 2A, the SSCPA of exon 4 of MK's β3 gene showed an abnormal faster migrating band. Direct cycle sequence analysis of PCR products from the patient and a normal control showed that the patient's DNA was homozygous for a T → G nucleotide substitution at nucleotide 445 resulting in a Leu117 → Trp mutation (Fig 2B). To confirm that MK was homozygous at this position, her PCR product was subcloned into a TA cloning vector and individual clones were sequenced. Of the six clones sequenced, all had the T → G mutation (data not shown).
Analysis of the patient's β3 gene. (A) SSCP analysis of β3 exon 4 in a normal control (lane 1), four unrelated thrombasthenic patients (lanes 2, 3, 5, and 6), and the patient (lane 4). The patient's lower band migrated faster than any of the other studied samples. (B) Sequence analysis of wild-type (WT) and the patient's (MK) DNA for β3 exon 4 showing a single T → G substitution in this exon.
Analysis of the patient's β3 gene. (A) SSCP analysis of β3 exon 4 in a normal control (lane 1), four unrelated thrombasthenic patients (lanes 2, 3, 5, and 6), and the patient (lane 4). The patient's lower band migrated faster than any of the other studied samples. (B) Sequence analysis of wild-type (WT) and the patient's (MK) DNA for β3 exon 4 showing a single T → G substitution in this exon.
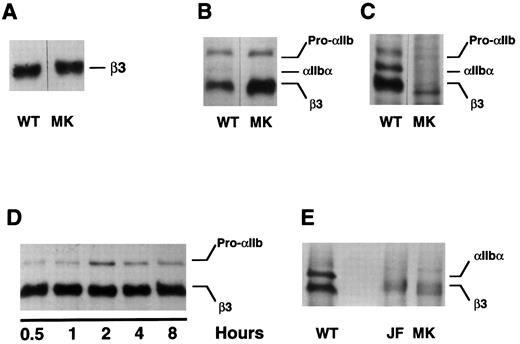
Expression of the mutant β3 allele in vitro. To confirm that the β3 Leu117 → Trp amino acid substitution was responsible for the patient's thrombasthenic phenotype, we introduced this mutation into the sequence of a wild-type β3 cDNA and expressed the mutant in COS-1 cells, either alone or with wild-type αIIb. Although the mutation did not demonstrably impair β3 synthesis (Fig 3A) or prevent the assembly of αIIbβ3 heterodimers (Fig 3B), the assembled heterodimers were not recognized by the MoAb A2A9 (Fig 3C), suggesting that the heterodimers were malfolded. To determine whether the abnormal folding affected αIIbβ3 stability, pulse-chase studies were performed. As shown in Fig 3D, the presence of β3 Leu117 → Trp had no obvious effect on the stability of αIIbβ3 over the 8-hour chase period.
Expression of β3 containing the Leu117 → Trp substitution in COS-1 cells. Wild-type (WT) and mutant (MK) β3 were expressed in COS-1, either alone or with αIIb. Cells were then either metabolically labeled with 35S-methionine or surface labeled with biotin-labeled proteins were immunoprecipitated by the antibodies indicated below and examined by SDS-polyacrylamide gel electrophoresis. (A) Wild-type and mutant β3 were expressed alone in COS-1 cells and immunoprecipitated from cells labeled with 35S-methionine using the β3-specific MoAb SSA6. (B and C) COS-1 cells were cotransfected with αIIb and either wild-type or mutant β3. After labeling the cells with 35S-methionine, αIIb-β3 was immunoprecipitated using the β3-specific MoAb SSA6 (B) or the heterodimer-specific MoAb A2A9 (C). (D) The cells were treated as in (B), but were pulse-chased for the various time intervals indicated. (E) The surface of COS-1 cells cotransfected with αIIb and either wild-type or mutant β3 was biotin labeled and immunoprecipitated using SSA6. WT is the wild-type complex, MK refers to αIIb-β3Leu117 → Trp complexes, and JF refers to αIIbGly273 → Asp-β3 complexes. Pro-αIIb corresponds to the single-chain αIIb precursor; αIIb-α corresponds to the αIIb heavy chain.
Expression of β3 containing the Leu117 → Trp substitution in COS-1 cells. Wild-type (WT) and mutant (MK) β3 were expressed in COS-1, either alone or with αIIb. Cells were then either metabolically labeled with 35S-methionine or surface labeled with biotin-labeled proteins were immunoprecipitated by the antibodies indicated below and examined by SDS-polyacrylamide gel electrophoresis. (A) Wild-type and mutant β3 were expressed alone in COS-1 cells and immunoprecipitated from cells labeled with 35S-methionine using the β3-specific MoAb SSA6. (B and C) COS-1 cells were cotransfected with αIIb and either wild-type or mutant β3. After labeling the cells with 35S-methionine, αIIb-β3 was immunoprecipitated using the β3-specific MoAb SSA6 (B) or the heterodimer-specific MoAb A2A9 (C). (D) The cells were treated as in (B), but were pulse-chased for the various time intervals indicated. (E) The surface of COS-1 cells cotransfected with αIIb and either wild-type or mutant β3 was biotin labeled and immunoprecipitated using SSA6. WT is the wild-type complex, MK refers to αIIb-β3Leu117 → Trp complexes, and JF refers to αIIbGly273 → Asp-β3 complexes. Pro-αIIb corresponds to the single-chain αIIb precursor; αIIb-α corresponds to the αIIb heavy chain.
It is noteworthy that throughout the chase αIIb was not cleaved into heavy and light chains (Fig 3D). Although this suggests that the mutant heterodimers were not exported from the endoplasmic reticulum to the Golgi complex in which αIIb cleavage occurs,37 αIIb cleavage is not required to express αIIbβ3 on the cell surface. Thus, to confirm that β3 Leu117 → Trp actually prevents the export of αIIbβ3 to the cell surface, we biotinylated the surface of COS-1 cells expressing wild-type αIIbβ3, αIIbβ3 containing β3 Leu117 → Trp, and αIIbβ3 containing αIIb Gly273 → Asp, a mutation known to impair the export of αIIbβ3 to the cell surface.16 We then immunoprecipitated αIIbβ3 using the β3-specific MoAb SSA6. Although labeled complexes were present on the surface of cells expressing wild-type αIIbβ3, none were not present on the surface of cells expressing either of the αIIbβ3 mutants (Fig 3E).
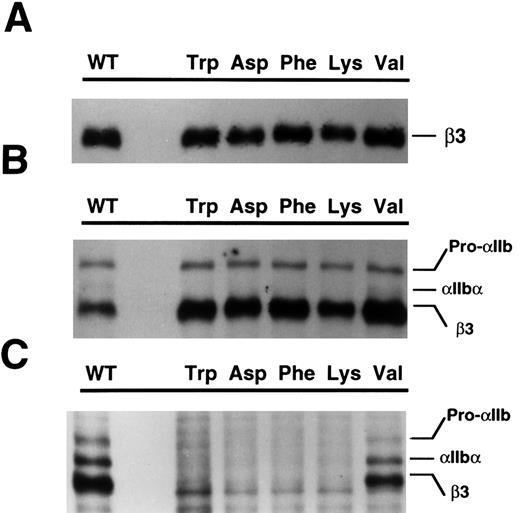
Effect of other mutations at β3 position 117 on the expression of αIIbβ3. The β3 mutation detected in patient MK resulted in the replacement of a highly conserved nonpolar Leu with a nonpolar Trp residue. To determine if the consequences of this mutation were due to the introduction of the bulky size indole side chain of Trp at this location in β3, we introduced other selected amino acids into position 117 by site-directed mutagenesis and examined their effect on αIIbβ3 expression in COS-1 cells. Phe and Val were selected as examples of nonpolar hydrophobic amino acids with differently sized side chains. The charged amino acids Asp and Lys were also selected because Leu117 precedes an array of charged and polar residues that have been implicated in both ligand and divalent cation binding to αIIbβ3.23 As shown in Fig 4A and B, the presence of Trp, Asp, Lys, Phe, or Val has no apparent effect on β3 synthesis (Fig 4A) or on the ability of β3 to associate with αIIb (Fig 4B). However, only heterodimers containing the wild-type Leu or Val at β3 position 117 could be immunoprecipitated using A2A9 (Fig 4C). Thus, these experiments indicate that, although a nonpolar residue is required at position 117 for proper β3 folding, there are limits to the size of the side chain of the residue that can be tolerated. Moreover, the presence of a charged residue at this position is not tolerated, regardless of the size of its side chain.
Expression of β3 containing additional β3117 substitutions in COS-1 cells. Transient expression studies in COS-1 cells were performed using either β3 mutants alone (A) or in combination with wild-type αIIb (B and C). In (A) and (B), the recombinant proteins were immunoprecipitated with SSA6 and in (C) with A2A9. The specific mutation introduced at β3117 is indicated for each lane. WT is the wild-type complex. Pro-αIIb corresponds to the single-chain αIIb precursor; αIIb-α corresponds to the αIIb heavy chain.
Expression of β3 containing additional β3117 substitutions in COS-1 cells. Transient expression studies in COS-1 cells were performed using either β3 mutants alone (A) or in combination with wild-type αIIb (B and C). In (A) and (B), the recombinant proteins were immunoprecipitated with SSA6 and in (C) with A2A9. The specific mutation introduced at β3117 is indicated for each lane. WT is the wild-type complex. Pro-αIIb corresponds to the single-chain αIIb precursor; αIIb-α corresponds to the αIIb heavy chain.
DISCUSSION
The basis for the Glanzmann thrombasthenia of the patient we studied was a Leu117 → Trp mutation in the β3 subunit of the integrin αIIbβ3. Although the mutation did not impair β3 synthesis or the assembly of αIIbβ3 heterodimers, it prevented export of the assembled heterodimers to the surface of transfected COS cells, presumably by causing their retention in a pre-Golgi compartment.38 We have observed that the ability to express αIIbβ3 on the COS cell surface is a sensitive index of the integrity of αIIbβ3 folding.22 Thus, it is likely that the impairment of αIIbβ3 expression by the Leu117 → Trp mutation results from a mutation-induced change in the conformation of the assembled heterodimer, a conclusion consistent with the failure of the mutant heterodimers to be recognized by the heterodimer-specific MoAb A2A9.
Leu117 is located near the proximal end of an array of polar and charged amino acids extending from Asp113 to Ser130 that has been implicated in ligand binding to αIIbβ339 and in low-affinity association of divalent cations with β340 (Fig 5). Furthermore, the region around amino acid 117 is highly conserved in the β3 of various species and in other β subunits such as β1 and β2.41 Based on homology modeling, it has been proposed that this region of β3 adopts a conformation similar to that of a metal ion-dependent adhesion site or “MIDAS” motif.42 However, in the absence of actual structural information about this region of β3, it is only possible to speculate about the structural consequences of mutations at position 117. Nevertheless, it is reasonable to assume from considerations of protein stability that the hydrophobic aliphatic side chain of Leu117 in this environment would be folded into the interior of β3. The inability to tolerate charged Asp or Lys residues at this position supports this assumption.43 Furthermore, there appears to be substantial steric constraint imposed on the folding of β3 at this position because the smaller side chain of Val, but not the bulky hydrophobic side chains of Trp and Phe, could be tolerated. In contrast, replacement of Asp119, just two residues carboxyl terminal to Leu117, with Tyr (the Cam β3 variant44 ) or alanine23 or replacement of Ser121, Ser123, Asp126, Asp127, and Ser130 with alanine23 neither affected αIIbβ3 expression nor the ability of the heterodimer to interact with the complex-specific MoAb 10E5. Identical results were observed in studies of two naturally-occurring mutations involving the Arg214 (Arg214 → tryptophen15 and Arg214 → glutamine14 ). Thus, the loss of charged or polar residues from these regions of β3 does not appear to significantly affect the folding of the protein. Because the side chains of these residues likely project from the exterior surface of β3 into the aqueous milieu, their presence would not be involved in the interior packing that appears to be critical for the final β3 conformation. This formulation is consistent with previous studies of the effect of amino acid substitutions on protein stability.43 These studies suggest that a only a limited number of residues in a protein contribute significantly to its folded structure. For example, it was observed in studies of the dimer interface of the DNA-binding domain of the λ repressor that the ability to tolerate amino acid substitutions correlated roughly with the accessibility of the wild-type side chain to solvent.45 Moreover, replacement of a major contributor to the hydrophobic core of T4 lysozyme, Ile 3, by Trp or Tyr had a substantial deleterious effect on lysozyme stability, as did the introduction of the polar or charged amino acids Ser, Thr, and Asp.46
Region of β3 affected by the Leu117 → Trp mutation. The amino acid sequence of the region of β3 extending from residues 113 to 130 is shown in the single letter code. Leu117 is designated by the darker arrow and Asp119 by the lighter arrow. The polar and charged amino acids mutated in Bajt and Loftus23 are underlined.
Region of β3 affected by the Leu117 → Trp mutation. The amino acid sequence of the region of β3 extending from residues 113 to 130 is shown in the single letter code. Leu117 is designated by the darker arrow and Asp119 by the lighter arrow. The polar and charged amino acids mutated in Bajt and Loftus23 are underlined.
The consequences of the Leu117 → Trp mutation in β3 identified in this patient are the same as those of four mutations involving the putative calcium binding region of αIIb.16,18-20 Like Leu117 → Trp, these mutations neither impair αIIb synthesis nor the assembly of αIIbβ3 heterodimers, but inhibit αIIbβ3 export to the cell surface. Moreover, they also specifically impair the recognition of αIIbβ3 by heterodimer-specific MoAbs, suggesting the possibility that the regions of αIIb and β3 containing these mutations physically interact to form the epitopes for these antibodies. Previous studies mapping the ligand binding region of αIIbβ3 with fibrinogen peptides support this possibility. It was found that peptides containing the sequence RGD, present at two places in the fibrinogen α chain, cross-link to a region of β3 encompassing amino acids 109-171,39 whereas peptides corresponding to the carboxyl terminus of the fibrinogen γ chain cross-link to a region of αIIb corresponding to its second putative calcium binding domain.47 Although both classes of peptides inhibited ligand binding to αIIbβ3, they did so in a mutually exclusive manner,48 suggesting that the ligand binding site on αIIbβ3 consists of a surface containing of segments of both αIIb and β3. A similar conclusion was reached by Loftus et al,49 who also found that the amino terminal third of αIIb defined the ligand specificity of αIIbβ3. It is noteworthy that β3 Leu117 → Trp and the αIIb calcium binding domain mutations reside either within or in proximity to the peptide cross-linking regions. Thus, it is possible that these mutations affect the conformation of the ligand binding surface, thereby explaining their effect on heterodimer-specific MoAb binding and supporting the notion that the segments of αIIb and β3 containing the mutations are physically associated.
In summary, we have identified a naturally occurring β3 mutation, Leu117 → Trp, which likely affects the conformation of αIIbβ3 and results in the intracellular retention of mutant heterodimers. Although Leu117 → Trp is located in immediate proximity to the previously described Cam mutation, Asp119 → Tyr, its consequences are markedly different because the latter had no effect of αIIbβ3 expression, but rather prevented ligand binding to the heterodimer. Thus, our results emphasize that the consequences of individual mutations in αIIb and β3 mutations, regardless of their location in the αIIb or β3 sequence, can be unique and are ultimately determined by their effect on subunit and heterodimer folding.
ACKNOWLEDGMENT
The authors thank Drs Simon and Margaret Karpatkin for generously sharing the patient presented in this report.
Supported in part by Grant No. HL40387 (to J.S.B. and M.P.), a grant from the Schulman Foundation (M.P.), and a grant from The Council for Tobacco Research-USA, Inc (#3152 to M.P.).
Address reprint requests to Mortimer Poncz, MD, The Children's Hospital of Philadelphia, 34th St and Civic Center Blvd, Philadelphia, PA 19104.