Abstract
The Wiskott-Aldrich syndrome (WAS) is a severe immunodeficiency and platelet deficiency disease arising from mutation(s) in the WASP gene, which in normal cells encodes an intracellular protein able to interact with other proteins relevant to the control of cytoskeleton organization. Immunodeficiency is mainly due to T-cell progressive malfunction. Salient defects of WAS T cells are a CD3-restricted impairment in proliferative responses and cytoskeletal abnormalities, including the frequent appearance of T cells with atypical morphology. We have investigated the possibility that the CD3-restricted defect and some of the cytoskeletal defects of WAS T cells are linked. For this purpose, we immortalized by means of infection with Herpesvirus Saimiri a number of previously described allospecific WAS T-cell lines. The resulting cells preserve the surface, molecular, and functional phenotypes of their parental lines, including a negligible WASP mRNA expression as well as the CD3-restricted defect and cytoskeleton abnormalities. Results show that, in CD3-stimulated WAS T cells, the pattern of temporal changes in cell shape and F-actin distribution is substantially different from that of control cells. Furthermore, polymerization of actin, a critical step in the CD3-mediated cytoskeleton reorganization, does not occur in WAS T-cell lines in response to OKT3 stimulation. In conclusion, our data link both CD3 and cytoskeletal defects in WAS T cells, strongly suggesting that cytoskeleton abnormalities are an underlying cause for WAS immunodeficiency.
THE WISKOTT-ALDRICH syndrome (WAS) is a complex, X-linked multiorganic condition, with a typical presentation of eczema, a reduced number and size of platelets, and a progressive immunodeficiency (reviewed in Rosen et al1 and Remold-O'Donnell et al2 ). Well-known defects in WAS peripheral lymphocytes include alterations in the expression of CD433 as well as a paucity in the microvilli observed by scanning electron microscopy (SEM) on the cell surface.4 Antibody-production abnormalities are subsidiary to inefficient T-lymphocyte function.5 T lymphocytes are severely affected, playing a central role in the pathogenesis of the disease.2 5
Therefore, considerable effort has been devoted towards the characterization of the precise functional defect(s) present in WAS T lymphocytes. The establishment of allospecific T-cell lines allowed us to identify a number of pathogenic events in the WAS.6 We also showed that the lack of microvilli and the appearance of aberrant cellular forms are characteristic of the disease, because they reflect deep alterations in the organization of the cytoskeleton.6 This is supported by the molecular structure of the mutated gene recently found in WAS patients (WASP ).7 The gene comprises 12 exons giving rise to a protein (WASp) of 502 amino acids of unknown function,7,8 but several groups have recently shown that WASp binds to the small GTPase protein Cdc42,9,10 a member of the Rho family of proteins that play a critical role in the cytoskeleton organization.11-13
However, the precise function of WASp is still unclear, and although no direct evidence has been obtained so far, it has been proposed that WASp may act as a key intracellular switch in several signaling transmission pathways.9,14-16 In this sense, we are aware of the potential complexity of WASp, because, besides Cdc42, a number of regulatory proteins bind to concrete domains of WASp, including the SH3-adaptor protein Nck and the T-cell receptor (TCR)-associated protein tyrosin kinase p59fyn and Tec family members Btk, Itk, Tec, Grb2, and phospholipase C. In the WASp sequence, potential binding sites exist for additional regulatory proteins, including a pleckstrin-homology domain and actin-regulating protein homology sequences.17-22 All of these proteins have important regulatory functions involved in proliferation, signal transduction, and/or organization of the cytoskeleton.
All cell lines belonging to the WAS allospecific panel and peripheral lymphocytes of most patients share a functional deficiency: T cells are unable to proliferate in response to mitogenic anti-CD3 monoclonal antibodies (MoAbs).23 We report in this work a specific defect in reorganization and polymerization of actin in WAS cells when challenged with anti-CD3 MoAbs. Because anti-CD3 responses require a distinctive reorganization of actin filaments24 and induction of WASP gene expression results in the formation of aggregates of WASp and polymerized actin,9 our data strongly suggest that T-cell defect(s) of cytoskeleton organization is a primary cause underlying WAS immunodeficiency.
MATERIALS AND METHODS
Culture media. For immortalization and culture of Herpesvirus Saimiri (HVS) Normal and WAS T-cell lines, a mixture (1:1) of RPMI 1640 (Bio-Whittaker, Verviers, Belgium) and CG (Serotec, Oxford, UK) was supplemented with 10% fetal calf serum, glutamine, penicillin-ampicillin, sodium pyruvate (all from Bio-Whittaker), 1 μmol/L 2-mercaptoethanol, and 50 UI/mL of human recombinant interleukin-2 (rIL-2; a generous gift of Hoffman-LaRoche, Nutley, NJ).
Antibodies. All antibodies for flow cytometry were fluorescein isothiocyanate (FITC)-labeled and purchased from Becton Dickinson (San José, CA), with the following exceptions: anti-CD25, CD28, and CD45RO were from Immunotech (Marseille, France); and unlabeled anti-CD43 MoAbs L10 and T305 were a kind gift of Dr Eileen Remold-O'Donnell (The Center for Blood Research, Boston, MA). Anti-CD3 OKT3 hybridoma cells were obtained from the American Type Culture Collection (Rockville, MD). Secreted antibodies contained in culture supernatants were precipitated with ammonium sulfate and affinity-purified using the Affi-Gel Protein-A system (Bio-Rad Laboratories, Hercules, CA). Before use, all batches of purified OKT3 MoAb were titrated in flow cytometry and tested for mitogenicity over nylon-wool–purified human T cells.
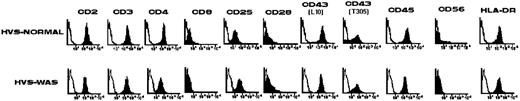
Surface phenotype of prototypic HVS-Normal (HVS-N8) and HVS-WAS (HVS-WAS1) immortalized T-cell lines. Flow cytometric analysis shows that HVS-Normal and HVS-WAS T-cell lines have similar surface expression of CD2, CD3, CD4, CD25, CD28, and CD43 isoforms (MoAb L10 recognizes tetrasaccharide and hexasaccharide forms of CD43, whereas MoAb T305 reacts exclusively with the hexasaccharide isoform of the molecule), CD45, and HLA-DR. The two cell lines shown in the figure and all other cell lines obtained are CD56−.
Surface phenotype of prototypic HVS-Normal (HVS-N8) and HVS-WAS (HVS-WAS1) immortalized T-cell lines. Flow cytometric analysis shows that HVS-Normal and HVS-WAS T-cell lines have similar surface expression of CD2, CD3, CD4, CD25, CD28, and CD43 isoforms (MoAb L10 recognizes tetrasaccharide and hexasaccharide forms of CD43, whereas MoAb T305 reacts exclusively with the hexasaccharide isoform of the molecule), CD45, and HLA-DR. The two cell lines shown in the figure and all other cell lines obtained are CD56−.
Immortalization of T cells. Normal and WAS T-cell lines were cultured as described,6 and 4 days before infection with HVS (kindly provided by Dr Bernhard Fleckenstein, University of Erlangen, Erlangen, Germany), cells were stimulated with the specific alloantigen expressed in the class II+ Raji B-cell line. At day 0, cells were washed, resuspended in RPMI/CG culture medium as indicated above, and cocultured with a 20% (vol/vol) fresh HVS crude supernatant produced in the epithelial owl monkey kidney cell line OMK as described.25 26 After an overnight incubation, cells were diluted with fresh media and expanded as required in 6-well plates (Nunc, Roskilde, Denmark), and 50% of media was replaced twice weekly. After 8 to 12 weeks, a steady growth of cells was evident and the cell line was considered immortalized. In all cases, HVS-immortalized T-cell lines remained dependent on the addition of exogenous IL-2 for continuous growth.
Flow cytometry. Cultured cells were washed and incubated with the indicated FITC-labeled MoAbs on ice for 30 minutes, washed again, and analyzed in a FACSort flow cytometer (Becton Dickinson). Incubation with unlabeled anti-CD43 MoAbs was followed by a second step with a goat antimouse (αIgG) FITC-antibody (Tago, Burlingame, CA).
Reverse transcription-polymerase chain reaction (RT-PCR) of WASP. Total RNA from HVS-Normal and HVS-WAS T-cell lines was extracted by the LiCl/urea method, and 4 μg of total RNA was reverse-transcribed using the AMV-Reverse transcription system (Promega, Madison, WI). Equal amounts of Normal and WAS cDNAs were amplified by PCR using 20-mers–specific primers deduced from the WASP sequence, spanning nucleotides 20-39 for the sense primer and 980-961 for the antisense primer. PCR amplification was performed in a Gene-Amp 9600 thermal cycler (Perkin-Elmer, Norwalk, CT) for 30 cycles with the following conditions: annealing at 60°C for 15 seconds, extension at 72°C for 75 seconds, and denaturing at 94°C for 20 seconds, with an initial denaturation of 2 minutes at 94°C and a final extension of 10 minutes at 72°C. Alliquots of PCR products were loaded onto 1.5% agarose gels and DNA stained with ethidium bromide.
Stimulation of cells. For CD3-mediated stimulation of cells, 100 ng of purified OKT3 MoAb per well, unless otherwise indicated, was adsorbed overnight to the culture plate in phosphate-buffered saline (PBS), pH 8.0, at 37°C. Cells used in all experiments were washed the day before and starved overnight in RPMI/CG medium supplemented with 2% fetal calf serum and deprived of rIL-2.
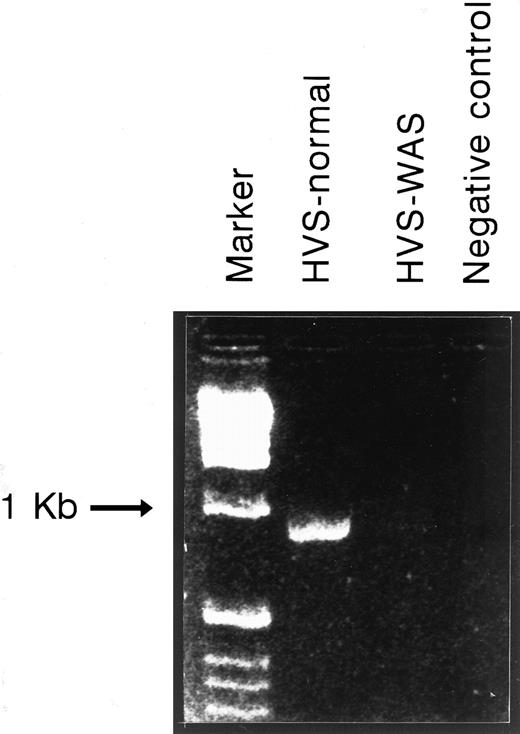
WASP mRNA expression of HVS-Normal (HVS-N8) and HVS-WAS (HVS-WAS1) immortalized T-cell lines as detected by RT-PCR. Equal amounts of total RNA extracted from indicated cell lines were reverse-transcribed as detailed in the Materials and Methods. cDNAs were amplified by PCR using a pair of primers spanning WASP sequence nucleotides 20-39 (forward) and 980-961 (reverse). Amplification of HVS-Normal cells yields the expected fragment of 900 bp, whereas no equivalent band is detected in the HVS-WAS1 cells. In the last lane, the PCR-negative control (no template) was loaded.
WASP mRNA expression of HVS-Normal (HVS-N8) and HVS-WAS (HVS-WAS1) immortalized T-cell lines as detected by RT-PCR. Equal amounts of total RNA extracted from indicated cell lines were reverse-transcribed as detailed in the Materials and Methods. cDNAs were amplified by PCR using a pair of primers spanning WASP sequence nucleotides 20-39 (forward) and 980-961 (reverse). Amplification of HVS-Normal cells yields the expected fragment of 900 bp, whereas no equivalent band is detected in the HVS-WAS1 cells. In the last lane, the PCR-negative control (no template) was loaded.
Proliferation assay. Proliferative responses of HVS-Normal and HVS-WAS immortalized T-cell lines were assessed as described.6 23 Briefly, T cells were harvested the day before the assay, washed, and cultured overnight in the absence of rIL-2. A total of 105 T cells per well were plated in a 96-well plate (Nunc) that had been precoated with the indicated amounts of OKT3 MoAb. Alternatively, cells were cocultured with different concentrations of Mitomycin-C–treated allospecific Raji cells. After 48 hours of culture, cells were labeled with 1 μCi/well of [3H]-thymidine (ICN, Costa Mesa, CA), the culture extended for an additional 16 hours and harvested and thymidine incorporation was finally quantified by liquid scintillation counting.
Proliferative responses of HVS-Normal (HVS-N8 [•]) and HVS-WAS (HVS-WAS1 [▵] and HVS-WAS7 [▴]) immortalized T-cell lines after stimulation with immobilized OKT3 MoAb and allospecific Raji cells. Overnight-starved T cells were plated (105 cells/well) in 96-well plates that had been precoated with 10, 25, 50, or 100 ng/well of OKT3 MoAb (left panel) or cocultured in the presence of 25, 50, or 100 × 103 mitomycin-C treated allospecific Raji cells (right panel). After 48 hours of culture, cells were labeled with [3H]-thymidine for an additional 16 hours. Thymidine incorporation was determined by liquid scintillation counting.
Proliferative responses of HVS-Normal (HVS-N8 [•]) and HVS-WAS (HVS-WAS1 [▵] and HVS-WAS7 [▴]) immortalized T-cell lines after stimulation with immobilized OKT3 MoAb and allospecific Raji cells. Overnight-starved T cells were plated (105 cells/well) in 96-well plates that had been precoated with 10, 25, 50, or 100 ng/well of OKT3 MoAb (left panel) or cocultured in the presence of 25, 50, or 100 × 103 mitomycin-C treated allospecific Raji cells (right panel). After 48 hours of culture, cells were labeled with [3H]-thymidine for an additional 16 hours. Thymidine incorporation was determined by liquid scintillation counting.
SEM. Exponentially grown cells were harvested from the culture and subjected to examination under the SEM as previously described for WAS T cells,6 with slight modifications. Briefly, Normal and WAS HVS-immortalized cells were washed in Ca2+/Mg2+-free PBS and allowed to bind to poly-l-lysine–coated slides simultaneously to the fixation process that was achieved during an overnight incubation at 4°C in an atmosphere saturated with glutaraldehyde vapors. Cells were postfixed in 1% osmium tetroxide for 1 hour, dehydrated with graded ethanols, subjected to critical point drying from carbon dioxide, and finally coated with gold. Samples were examined on a Zeiss DSM 950 Scanning electron microscope (Zeiss, Oberkochen, Germany).
Staining of filamentous actin. For visualization of actin reorganization, 5 × 105 cells/well were stimulated with precoated OKT3 in 8-well chamber slides (Nunc). The reactions were stopped by fixing the cells in 2% paraformaldehyde (Sigma Chemical, St Louis, MO) for 30 minutes at room temperature. After washes with PBS, slides were incubated with tetramethilrodamine isothyocianate (TRITC)-labeled phalloidin (Molecular Probes, Eugene, OR; 1:50 dilution in PBS/0.05% saponin [Sigma]) for 40 minutes at room temperature. Cells were subsequently washed in PBS, the chambers were removed, and the slides were air-dried. Coverslips were mounted over 100 μL of a 1:1 solution of PBS/glycerol containing 10% water saturated with the antioxidant p-phenylene diamine (Sigma). Slides were viewed and photographed immediately. For the analysis by flow cytometry of CD3-induced actin polymerization, cells were stimulated in OKT3-coated 24-well plates as indicated above, fixed, and stained with rhodamine-phalloidin as before. After washes, cells were resuspended in PBS and analyzed after appropriate gating of cells in the FACSort flow cytometer. If saturating quantities of fluorescent phalloidin are used, then the fluorescence is a quantitative measure of the amount of filamentous actin in cells.27 Where indicated, cells were pretreated for 45 minutes at 37°C with 3 μmol/L cytochalasin D (Sigma) and stimulated with MoAbs immediately afterwards. Phalloidin is an agent that selectively binds to F-actin, whereas cytochalasin D binds to the barbed end of actin filaments, inhibiting both the association and dissociation of subunits at that end.27
RESULTS
Immortalization of WAS-allospecific T-cell lines by infection with HVS. To improve our experimental system of allospecific T-cell lines, a number of Normal and WAS T-cell lines underwent selective immortalization by means of HVS infection.25 26 Immortalization was achieved 4 to 6 weeks after infection, when a vigorous pace of growth is observed in the infected cells. All of our HVS cell lines (WAS and Normal) remain stable after long-term culture and continue their dependence on the addition of exogenous IL-2 for continuous culture.
Surface phenotype of HVS-immortalized WAS and Normal T-cell lines. Staining of cells with specific MoAbs showed by immunocytometric analysis a surface phenotype identical to that of their corresponding parental cell lines.6 This includes equivalent expression of CD2, CD3, CD4 or CD8, CD25, CD28, CD43 isoforms, CD45, and HLA-DR antigens (Fig 1). It has been described that HVS-immortalized T cells occasionally acquire the expression of CD56.25 All our cell lines are CD56−.
WASP mRNA expression in Normal and WAS HVS-immortalized T-cell lines. Expression of WASP mRNA in Normal and WAS HVS-immortalized T-cell lines was studied by RT-PCR as detailed in the Materials and Methods. Amplification of the expected fragment of 900 bp was achieved in the HVS-Normal line, whereas no specific band was observed in the HVS-WAS T-cell line (Fig 2). Similar results were obtained using a different pair of internal WASP primers (data not shown).
Functional phenotype of HVS-WAS and HVS-Normal T-cell lines. A restricted defect in the CD3-mediated proliferative response was identified in WAS peripheral lymphocytes and in the panel of allospecific WAS T-cell lines.23 To verify the fidelity of the functional phenotype of the HVS-immortalized T-cell lines, Normal and WAS T cells were challenged with immobilized anti-CD3 MoAb. The coupling of the CD3 complex by the OKT3 MoAb elicits a vigorous proliferative response in Normal, but not in WAS, HVS-immortalized cells (Fig 3, left panel). Both Normal and WAS lines strongly proliferate when stimulated with the specific alloantigen expressed in the Raji B-cell line (Fig 3, right panel). This pattern of responses precisely mirrors that of the untransformed parental T-cell lines, thereby indicating the functional preservation of the clonotypic TCR.
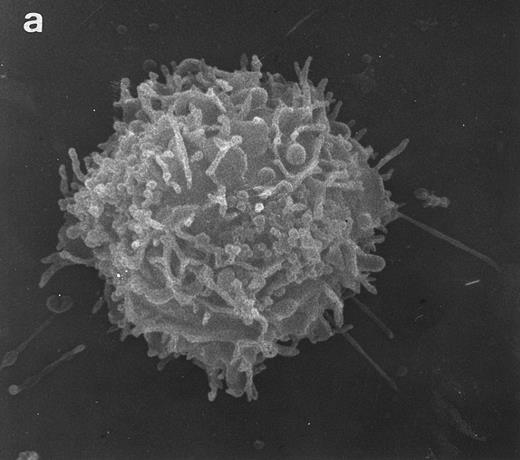
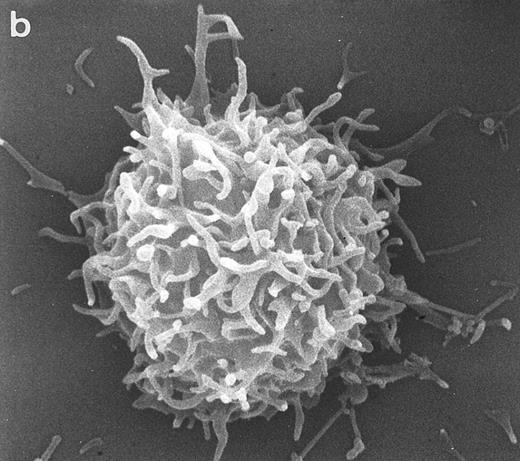
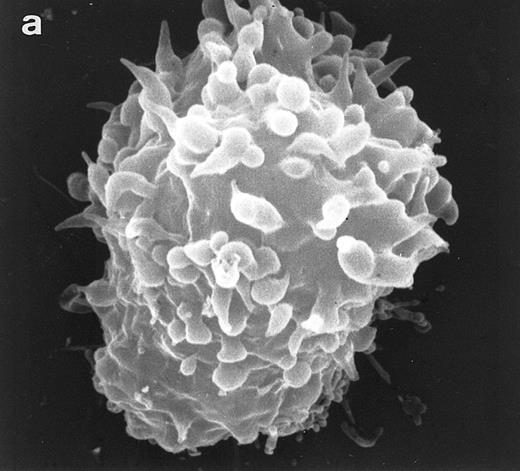
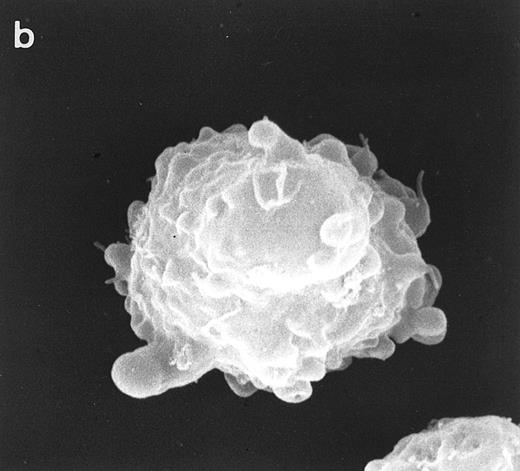
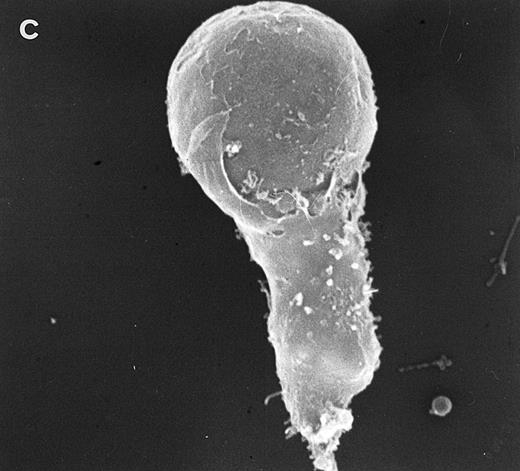
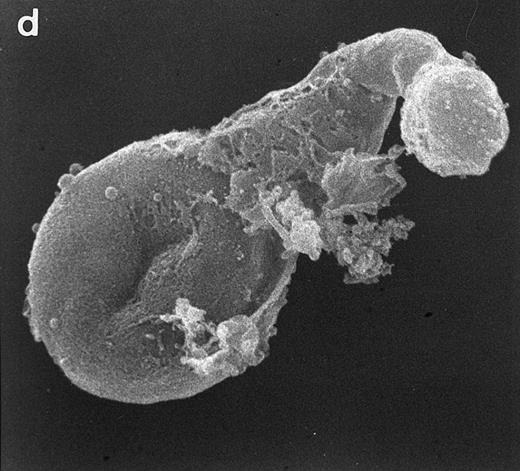
Surface morphology of T-cell lines. As mentioned in the introduction, SEM analysis of WAS cells has been extensively used to substantiate cytoarchitectural defects.4,6 The examination of HVS-Normal T cells showed cell populations relatively homogeneous in size and morphology, and virtually all cells display the dense projection of surface microvilli characteristic of peripheral blood lymphocytes and allospecific T-cell lines obtained from normal individuals. Figure 4 shows random individual cells. In contrast, the size and morphology of the WAS T-cell lines are very heterogeneous, showing prominent defects. The previously observed cells with bulbous or lamellar forms are represented in Fig 5a and b. The cells with elongated and aberrant shapes are depicted in Fig 5c, d, and e. The described defects appear to be in the HVS-WAS cells more consistent and conspicuous than in the previously described long-term allospecific T-cell lines and/or WAS peripheral T cells.4 6
SEM of HVS-Normal (HVS-N8) T cells. Random individual cells of an HVS-immortalized T-cell line derived from a normal individual. Cells are homogeneous in size and morphology, displaying dense projections of surface microvilli that are typical of normal peripheral blood lymphocytes and allospecific normal T-cell lines. (Original magnification × 8,000.)
SEM of HVS-Normal (HVS-N8) T cells. Random individual cells of an HVS-immortalized T-cell line derived from a normal individual. Cells are homogeneous in size and morphology, displaying dense projections of surface microvilli that are typical of normal peripheral blood lymphocytes and allospecific normal T-cell lines. (Original magnification × 8,000.)
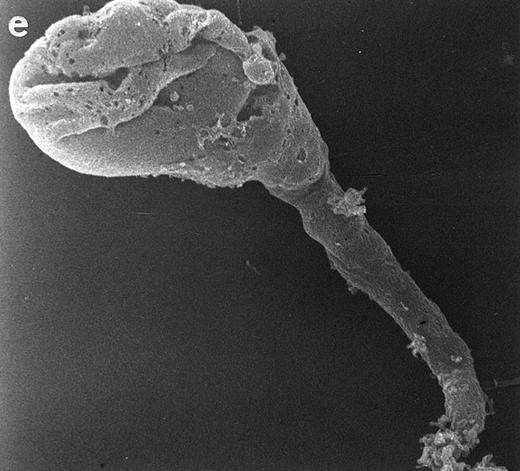
Scanning electron micrographs of HVS-immortalized T cells from a WAS patient (HVS-WAS1). The WAS T-cell lines are heterogeneous in size and morphology, showing all cells' conspicuous abnormalities. Cells with lamellar or bulbous morphology are represented in (a) and (b). Cells with elongated and aberrant shapes are depicted in (c), (d), and (e). (Original magnification × 8,000.)
Scanning electron micrographs of HVS-immortalized T cells from a WAS patient (HVS-WAS1). The WAS T-cell lines are heterogeneous in size and morphology, showing all cells' conspicuous abnormalities. Cells with lamellar or bulbous morphology are represented in (a) and (b). Cells with elongated and aberrant shapes are depicted in (c), (d), and (e). (Original magnification × 8,000.)
Differential CD3-dependent reorganization of actin filaments in HVS-WAS cells. The coupling of the TCR/CD3 molecular complex triggers a distinct pattern of reorganization of the cellular F-actin.24,28 Therefore, we examined the response of the cytoskeleton after challenging with anti-CD3 MoAb HVS-Normal and HVS-WAS T-cell lines. In HVS-Normal cells, the actin reorganization in response to anti-CD3 stimulation is similar to that described for Jurkat cells.24 The early phase of the response (5 minutes) shows a diffuse distribution of the F-actin throughout the cytoplasm, with a reinforcement adjacent to the inner membrane of the cell that conforms the typical actin rings (Fig 6a). The remodeling of the actin cytoskeleton progresses with time, and spreading of cells is accompanied by the appearance of attachment pseudopods at 15 and 30 minutes after stimulation (Fig 6b and c).
Actin reorganization of HVS-Normal (HVS-N8 [a through d]) and HVS-WAS (HVS-WAS1 [e through h]) T-cell lines in response to stimulation with immobilized anti-CD3 MoAb. HVS-Normal (a through d) and HVS-WAS (e through h) T cells were stimulated with immobilized OKT3 MoAb for 5 minutes (a and e), 15 minutes (b and f ), and 30 minutes (c and g). In HVS-Normal cells, actin rings are progressively dissolved, followed by cellular spreading and frequent formation of pseudopodia. In contrast, in HVS-WAS T cells, actin rings yield to strongly polarized accumulations of F-actin. HVS-WAS T cells that fail to spread acquire atypical shapes with the appearance of structures resembling uropods. Pretreatment of cells with cytochalasin D abrogates all actin-specific changes in both HVS-Normal (d) and HVS-WAS (h) immortalized T cells. (Original magnification × 400, except for [d] and [h], which are ×630.)
Actin reorganization of HVS-Normal (HVS-N8 [a through d]) and HVS-WAS (HVS-WAS1 [e through h]) T-cell lines in response to stimulation with immobilized anti-CD3 MoAb. HVS-Normal (a through d) and HVS-WAS (e through h) T cells were stimulated with immobilized OKT3 MoAb for 5 minutes (a and e), 15 minutes (b and f ), and 30 minutes (c and g). In HVS-Normal cells, actin rings are progressively dissolved, followed by cellular spreading and frequent formation of pseudopodia. In contrast, in HVS-WAS T cells, actin rings yield to strongly polarized accumulations of F-actin. HVS-WAS T cells that fail to spread acquire atypical shapes with the appearance of structures resembling uropods. Pretreatment of cells with cytochalasin D abrogates all actin-specific changes in both HVS-Normal (d) and HVS-WAS (h) immortalized T cells. (Original magnification × 400, except for [d] and [h], which are ×630.)
In contrast, WAS cells show deep abnormalities in the pattern of actin reorganization. Thus, whereas in some cells actin rings are readily observed after 5 minutes of OKT3 stimulation (Fig 6e), in other cells the actin begins to cluster in a cellular pole. The striking polarization of F-actin becomes a generalized phenomena after 15 minutes of stimulation (Fig 6f and g). Simultaneously, cells display aberrant morphologies and are unable to undergo the process of spreading that is observed in the HVS-Normal cells. Pretreatment of cells with cytochalasin D prevents all CD3-mediated specific changes in cell shape (Fig 6d and h). At the same time, nonstimulating MoAbs do not induce any of the CD3-associated changes described above (data not shown).
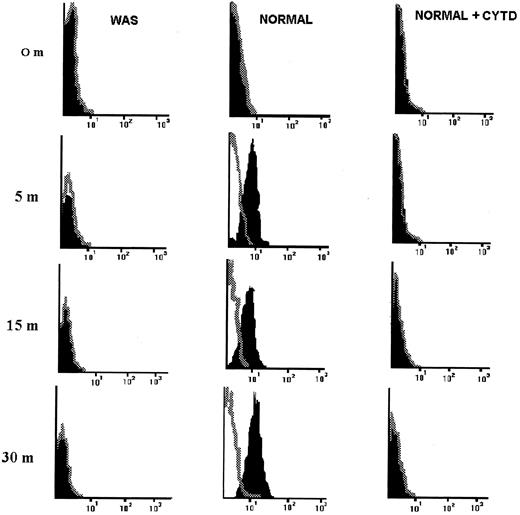
Defective polymerization of actin in HVS-WAS cells in response to anti-CD3 challenge. It is known that polymerization of actin is a critical step in the CD3-mediated cytoskeleton reorganization.24,28 Based on the described observations, we have examined the ability of HVS-Normal and HVS-WAS T-cell lines to form new actin filaments upon OKT3 engagement of the CD3/TCR complex. A rapid (5 minutes) polymerization of actin occurs in HVS-Normal cells as determined by phalloidin binding changes detected in flow cytometry (Fig 7). The fluorescence intensity increases until 30 minutes after stimulation, reflecting the kinetic of the process of actin polymerization, specifically abrogated after pretreatment of HVS-Normal cells with cytochalasin D. In sharp contrast, no differences were found in fluorescence staining intensity when OKT3-stimulated and nonstimulated HVS-WAS cells were compared, indicating that HVS-WAS cells are unable to polymerize actin in response to OKT3 challenge. Interestingly, stimulation of HVS-Normal and WAS cells with phorbol myristate acetate (PMA) does not induce new formation of actin filaments (data not shown). PMA induces polymerization of actin in resting peripheral lymphocytes,28 but has no effects on the Jurkat T-cell line.24
HVS-immortalized WAS T cells do not polymerize actin in response to stimulation with immobilized anti-CD3 MoAb. HVS-Normal (HVS-N8) and HVS-WAS (HVS-WAS1) cells were stimulated with immobilized anti-CD3 MoAb for indicated times, fixed, permeabilized, stained with Rhodamine-phalloidin, and finally analyzed by flow cytometry. Fluorescence profiles of stimulated (solid figures) and unstimulated (open figures, dotted lines) cells are overlayed. Thus, the negative control is set to represent the total amount of F-actin contained in unstimulated cells. The increase in the fluorescence intensity indicates a rapid passage from G-actin to F-actin in Normal cells in response to OKT3 (central column), a process that is specifically abrogated by pretreatment of cells with cytochalasin D (right column). HVS-WAS cells are unable to polymerize actin in response to OKT3 (left column).
HVS-immortalized WAS T cells do not polymerize actin in response to stimulation with immobilized anti-CD3 MoAb. HVS-Normal (HVS-N8) and HVS-WAS (HVS-WAS1) cells were stimulated with immobilized anti-CD3 MoAb for indicated times, fixed, permeabilized, stained with Rhodamine-phalloidin, and finally analyzed by flow cytometry. Fluorescence profiles of stimulated (solid figures) and unstimulated (open figures, dotted lines) cells are overlayed. Thus, the negative control is set to represent the total amount of F-actin contained in unstimulated cells. The increase in the fluorescence intensity indicates a rapid passage from G-actin to F-actin in Normal cells in response to OKT3 (central column), a process that is specifically abrogated by pretreatment of cells with cytochalasin D (right column). HVS-WAS cells are unable to polymerize actin in response to OKT3 (left column).
DISCUSSION
Actin is a major cytoskeletal protein that is found in resting cells in two forms: a monomeric, globular form (G-actin) and a polymerized, filamentous form (F-actin). A critical step in the T-cell response to antigen and CD3-mediated stimulation is the polymerization of actin.24,29 Furthermore, activation of T cells with anti-CD3 MoAbs provokes a distinctive pattern of F-actin reordenation in the cell as well as characteristic changes in cell shape. This includes the progressive dissolution of actin rings, which is followed by cellular spreading and frequent formation of pseudopodia.24 Thus, we explored the possibility that actin reorganization abnormalities could provide a common ground for the previously reported defects in WAS T cells, namely the impaired CD3-mediated response and the morphologic defects.
The establishment of a panel of allospecific WAS T-cell lines has been a useful tool for dissecting functional defects present in WAS T lymphocytes. This included the re-evaluation of the biologic significance of the CD43 abnormalities and the finding of a restricted defect in CD3-mediated proliferative responses.6,23 The presence in the system of allostimulatory cells, which are needed for the growth of our T-cell lines, may hamper the interpretation of results that specifically concern to ubiquitous cellular components. Thus, pertaining to the present study, elimination of all non–T-cell elements would be desirable, provided that the resulting cells retain the morphologic, phenotypical, molecular, and functional properties of the parental cells from which they are derived. With this aim in mind, we have undertaken a novel approach in the field of primary immunodeficiencies, based on the immortalization of T cells with the HVS, a new world monkey virus that selectively infects human T cells in vitro.25 26
The results obtained in this work show that all HVS-Normal and HVS-WAS T cells are faithful counterparts of their parental lines, because they have a surface phenotype that is identical to that of the cell lines from which they are derived. The distinctive pattern of responses is also preserved, as determined by the proliferative responses to allospecific stimulatory cells and to anti-CD3 MoAbs. In addition, examination of HVS-WAS T-cell lines under SEM shows conspicuous morphologic abnormalities that are typical to WAS T cells, such as the lack of microvilli from the membrane and the presence of aberrant cellular forms.6 Therefore, our results show the adequacy of HVS-immortalization as a valid experimental approach to study, in the absence of other cell types, intrinsic T-cell abnormalities in WAS and other immunodeficiencies.
The stimulation with anti-CD3 MoAb of the HVS-Normal and WAS T cells shows striking defects in the latter. First, HVS-WAS T cells retain the CD3-restricted defect in proliferative response. Second, the changes in actin reorganization and cell shape that follows OKT3 stimulation of HVS-Normal T cells are not observed in HVS-WAS T cells. Indeed, in HVS-WAS T cells, actin rings yield to strongly polarized accumulations of F-actin. HVS-WAS T cells, which fail to spread, acquire atypical shapes with the appearance of structures resembling uropods. Third, in HVS-Normal cells, OKT3 binding gives rise to a rapid passage from G-actin to F-actin, as shown in flow cytometry by the increased staining of cells with TRITC-phalloidin. Strikingly, the CD3-induced actin polymerization is not detectable in HVS-WAS cells, because Rhodamine-phalloidin staining intensity is the same in stimulated and nonstimulated cells in all timepoints studied.
An immediate implication of this finding is that the abnormal pattern of actin reorganization in the HVS-WAS cells occurs exclusively at the expenses of the F-actin existing in the cell before stimulation. In fact, WASp, which is deficient in HVS-WAS T cells, putatively binds actin through regions on its carboxy-terminal end with some structural similarities to known actin-regulating proteins. Consequently, the possibility that WASp plays a direct role in the process of polymerization of actin from the globular to the filamentous form has to be considered. Supporting this idea is the fact that, in genetically altered WASp-overexpressing cells, the protein colocalizes with polymerized actin in the cytoplasm, in an interaction that is regulated by Cdc42.9
In this context, WAS can be seen as a natural knock-out model of WASP, and our data indicate that the product of this gene has a critical role in cytoskeleton reordenation events. Other potential functions of WASp are suggested by its capacity to bind a number of SH3-proteins (ie, p59fyn, Nck, etc) mainly involved in transmission of activation and proliferation signals. Because WAS T cells (parental and immortalized) do proliferate, the participation of WASp in controlling cell proliferation could be either a negative regulatory role or otherwise a dispensable function.
As a corollary, our data raise some clues for deciphering the intriguing WAS paradox, namely the coexistence of a defective CD3-mediated intracellular signaling with a normal allogeneic response. In this regard, it has been shown that Cdc42 is responsible for the cytoskeletal remodeling leading to polarization of T cells toward the direction of a external signal, delivered by antigen-presenting cells in specific responses.29 However, it remains unknown whether the same occurs during T-lymphocyte allorecognition. Thus, a possibility to consider is that, in WASp-deficient cells at least, CD3-transduced extracellular signals proceed to the cytoplasm following an intracellular route in which WASp is a checkpoint. Accordingly, in the alloresponse, in which a number of ligand-receptor pair interactions occur, productive cytoskeleton rearrangement would be controlled by alternative pathways that are able to replace WASp function. This hypothesis would also explain the unusually high rate of rejection of haploidentical grafts in bone marrow transplanted WAS patients.30-33
A number of issues regarding the precise role of WASp in the regulation of T-cell function still remain unresolved. Nevertheless, our data provide the first evidence of a direct connection between two relevant defects of WAS T cells, namely the CD3-restricted defect and actin reorganization abnormalities, strongly suggesting that an underlying cause for WAS immunodeficiency is an anomalous cytoskeleton structure.
ACKNOWLEDGMENT
The authors are indebted to Dr Juan M. Millán for help with the infection and establishment of HVS-immortalized T-cell lines; to Alicia González and Concha Hernández (Centro de Instrumentación Cientı́fica of the University of Granada, Granada, Spain) for expert assistance with scanning electron microscopy techniques; to Dr Bernhard Fleckenstein for providing us with the Herpesvirus Saimiri and the OMK cells; to Hoffman-LaRoche for their generous gift of human rIL-2; and to Dr Eileen Remold-O'Donnell for reagents and help.
Supported by Grant No. PB94-0753 from the DGICYT of the Spanish Ministry of Education and Science and Grant No. FIS 95-1170 from the Fondo de Investigaciones Sanitarias. M.D.G. is a predoctoral fellow of the Spanish Ministry of Education.
Address reprint requests to Manuel Santamarı́a, MD, Departamento de Fisiologı́a e Inmunologı́a, Facultad de Medicina, Avda. Menéndez Pidal s/n, E-14004 Córdoba, Spain.

![Fig. 3. Proliferative responses of HVS-Normal (HVS-N8 [•]) and HVS-WAS (HVS-WAS1 [▵] and HVS-WAS7 [▴]) immortalized T-cell lines after stimulation with immobilized OKT3 MoAb and allospecific Raji cells. Overnight-starved T cells were plated (105 cells/well) in 96-well plates that had been precoated with 10, 25, 50, or 100 ng/well of OKT3 MoAb (left panel) or cocultured in the presence of 25, 50, or 100 × 103 mitomycin-C treated allospecific Raji cells (right panel). After 48 hours of culture, cells were labeled with [3H]-thymidine for an additional 16 hours. Thymidine incorporation was determined by liquid scintillation counting.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/90/8/10.1182_blood.v90.8.3089/4/m_bl_0026f3.jpeg?Expires=1769113670&Signature=AyB7fA1sCw8sgXj~DaGKIZelgereqLC76QdxAMWET69YKiWD0pgPyt9vRPwhEhlGGuqN5~Y5UxQxfoezmUhZr5GzNORKflwcxAhR1c5v6ZmnvcrNJRBry8NXJauO--Yzs2gMiaYCXgnAWazgb8ZqOdxGxlx8FN4HrdHYDOmPLZBKDh59R~oycyITxb6GN78dw6WMx6Lp6XNxoFqvCThjtvlP1r40ZNyT2Lq4SIlP51WbogiOhZakTcVOxcnQCKq2q4RmXtNt6WOe9lG3hGeb0EsEUP7YjWaHu3nmR6TmiEeHx9vBBYAYJrf~kRohr9zaNQ5V5BqmGXzXP~g~irl1Zw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 6. Actin reorganization of HVS-Normal (HVS-N8 [a through d]) and HVS-WAS (HVS-WAS1 [e through h]) T-cell lines in response to stimulation with immobilized anti-CD3 MoAb. HVS-Normal (a through d) and HVS-WAS (e through h) T cells were stimulated with immobilized OKT3 MoAb for 5 minutes (a and e), 15 minutes (b and f ), and 30 minutes (c and g). In HVS-Normal cells, actin rings are progressively dissolved, followed by cellular spreading and frequent formation of pseudopodia. In contrast, in HVS-WAS T cells, actin rings yield to strongly polarized accumulations of F-actin. HVS-WAS T cells that fail to spread acquire atypical shapes with the appearance of structures resembling uropods. Pretreatment of cells with cytochalasin D abrogates all actin-specific changes in both HVS-Normal (d) and HVS-WAS (h) immortalized T cells. (Original magnification × 400, except for [d] and [h], which are ×630.)](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/90/8/10.1182_blood.v90.8.3089/4/m_bl_0026f6a.jpeg?Expires=1769113670&Signature=DDL6kS-r6ikjd6K6Ud3PYEjZf7CEp3~epomVW09VlpPQ3PR~7eFGtEtojmBn5RSU4aHoVqA04HXgvmTGIcSptYcdLFV1BRld86TKNQsmRknYT0FVs1YWA7DD9XlalRL0IN0BQ7Wd9yWJ95LE3os4FcV9dOoqilZwDPCr2jnmoKsdEGhRMQ6-ll~yDK4VaXAm~VklZ-xDwkyaS2z7RxZQ4RMAE-dlkTC9vmGomEAwqNGfkmXRf7b1836QvWpKNIJf90pX8j7TmDcWYWO-veNOCiNl7SQfDdBFlFhleulny5PHImbE~3BjQNqSVhq1YcokewuSVuD9sOhML0Nz8zq~kw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 6. Actin reorganization of HVS-Normal (HVS-N8 [a through d]) and HVS-WAS (HVS-WAS1 [e through h]) T-cell lines in response to stimulation with immobilized anti-CD3 MoAb. HVS-Normal (a through d) and HVS-WAS (e through h) T cells were stimulated with immobilized OKT3 MoAb for 5 minutes (a and e), 15 minutes (b and f ), and 30 minutes (c and g). In HVS-Normal cells, actin rings are progressively dissolved, followed by cellular spreading and frequent formation of pseudopodia. In contrast, in HVS-WAS T cells, actin rings yield to strongly polarized accumulations of F-actin. HVS-WAS T cells that fail to spread acquire atypical shapes with the appearance of structures resembling uropods. Pretreatment of cells with cytochalasin D abrogates all actin-specific changes in both HVS-Normal (d) and HVS-WAS (h) immortalized T cells. (Original magnification × 400, except for [d] and [h], which are ×630.)](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/90/8/10.1182_blood.v90.8.3089/4/m_bl_0026f6b.jpeg?Expires=1769113670&Signature=EaUNUz5CTJKL6XgCGOEXVAVIzik2fzhExVR6ImMK4yMz3hy7vDwdXSvqngUpmsC89tBxWG5Y4om3k3Mq16VWTICIUOhC7gd84QH9qvHrGV-um5vuYpiuIDV-3l28UTPk03KG0GPyrAlzqBvGXCukVZCuXe8uRTCzg1SSgryT3ejnRnRt6JJ8OynJAH1p0azUHbtmTNDSdgfPh9o2GMvaJbJlmDKqmC2-s1Yz90pAwbQ9ckdFfhM6Zzx6eaYDLq7rc7yzVSWsTZGbZL94fSYeMvYRwYNjtAn9oJZQNmGsep2~62JSy~OZQeq9mEFJ5HyyJIUz1r~NVeMdY8~VnI0bpw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 6. Actin reorganization of HVS-Normal (HVS-N8 [a through d]) and HVS-WAS (HVS-WAS1 [e through h]) T-cell lines in response to stimulation with immobilized anti-CD3 MoAb. HVS-Normal (a through d) and HVS-WAS (e through h) T cells were stimulated with immobilized OKT3 MoAb for 5 minutes (a and e), 15 minutes (b and f ), and 30 minutes (c and g). In HVS-Normal cells, actin rings are progressively dissolved, followed by cellular spreading and frequent formation of pseudopodia. In contrast, in HVS-WAS T cells, actin rings yield to strongly polarized accumulations of F-actin. HVS-WAS T cells that fail to spread acquire atypical shapes with the appearance of structures resembling uropods. Pretreatment of cells with cytochalasin D abrogates all actin-specific changes in both HVS-Normal (d) and HVS-WAS (h) immortalized T cells. (Original magnification × 400, except for [d] and [h], which are ×630.)](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/90/8/10.1182_blood.v90.8.3089/4/m_bl_0026f6c.jpeg?Expires=1769113670&Signature=ZnBai13oqSITUSXV~IYs8rDMM4lgBnDOBgdXDFWp8NhGoDrUiUuzG~3psaA0qtpmO6XxvFWQleMFSZEkfya79o-jfU8IViixbLEREVXz4u6XsfR2J~Y3RiBX4OLI-cjcCHbblBpBIdYYR09Gw0M9y3EWA6AkvIackNuoYYHv664rMiBhq-BpQPSWHF3hMDnb0PKWawSXTQE3Vet68cDxC9kjwb2qHcpiT8xPfGMRen2RCRr2XExcZYYGq2zCHs7m8V6ae6L6Yfr4M12Y2a3~q9nyb9Jo2EqijenddNMW2zMzeD1p4L20Ds5rESWhCXwJIsSB9qiQgDVhyWobEA7A-A__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 6. Actin reorganization of HVS-Normal (HVS-N8 [a through d]) and HVS-WAS (HVS-WAS1 [e through h]) T-cell lines in response to stimulation with immobilized anti-CD3 MoAb. HVS-Normal (a through d) and HVS-WAS (e through h) T cells were stimulated with immobilized OKT3 MoAb for 5 minutes (a and e), 15 minutes (b and f ), and 30 minutes (c and g). In HVS-Normal cells, actin rings are progressively dissolved, followed by cellular spreading and frequent formation of pseudopodia. In contrast, in HVS-WAS T cells, actin rings yield to strongly polarized accumulations of F-actin. HVS-WAS T cells that fail to spread acquire atypical shapes with the appearance of structures resembling uropods. Pretreatment of cells with cytochalasin D abrogates all actin-specific changes in both HVS-Normal (d) and HVS-WAS (h) immortalized T cells. (Original magnification × 400, except for [d] and [h], which are ×630.)](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/90/8/10.1182_blood.v90.8.3089/4/m_bl_0026f6d.jpeg?Expires=1769113670&Signature=kt-5mOIFjdAjwnDDYwE4QrIu0IOu9MwPoYZTaXRfNMWEV-o1t9o6eRQb5b923Q8r20weZgfnDv1CSvLG9q~JWQAXUL9ZU9WDc6mlLEXVt1qlEiLKuJ7c9CwZGCrKoRevKiCfMAlrASvE3bn64STNEmfcVVU0uhPZZ8mwLScEu3t3xufNHxuRxmvbe1GZ2qV5VYxjzJ1uL1D3xwx~H7TvegiNtoLSeli5Zx-P6XbSlURpA7Ua2GWT8SHj8DvCbRt39a~rPKUv0FF0EvfDHBsa5hbYO~bNvOJ70mycLhiXd-HL7VM5z4oxTG6AnE-6J4yRwHYaRx8Vp3AmX9-VYwmmNA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 6. Actin reorganization of HVS-Normal (HVS-N8 [a through d]) and HVS-WAS (HVS-WAS1 [e through h]) T-cell lines in response to stimulation with immobilized anti-CD3 MoAb. HVS-Normal (a through d) and HVS-WAS (e through h) T cells were stimulated with immobilized OKT3 MoAb for 5 minutes (a and e), 15 minutes (b and f ), and 30 minutes (c and g). In HVS-Normal cells, actin rings are progressively dissolved, followed by cellular spreading and frequent formation of pseudopodia. In contrast, in HVS-WAS T cells, actin rings yield to strongly polarized accumulations of F-actin. HVS-WAS T cells that fail to spread acquire atypical shapes with the appearance of structures resembling uropods. Pretreatment of cells with cytochalasin D abrogates all actin-specific changes in both HVS-Normal (d) and HVS-WAS (h) immortalized T cells. (Original magnification × 400, except for [d] and [h], which are ×630.)](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/90/8/10.1182_blood.v90.8.3089/4/m_bl_0026f6e.jpeg?Expires=1769113670&Signature=AAVqIAsK4Au4dyJ9OMiGHBFPGBkSI7XdeSmaubqHGbF9mc8dcTm-p~qyYzIDSfWqwLr5QTNXetAZeGmVuCKnYZ99GMU7vKIjjjMx6SimUBbEwwC~~dGfbQIrrKXBktME2SPYZGys4rejIKyBCmvQT8X1OC36A0dhxJ8mqZiNSDD3GnW6bJ602qGar0dwCqcIW7WBapWJssKqh5w4ZFjMb6xP5qa9Beha4wXTFsE7iplEai7ZWYj39ox7BgmzL1PIfyWmE4uLuP9WUUlQk-3cVYg6wN6YJdgmVzBhMH9GTPiKJdntlvMH4tLc5QdLHRZF6OdITIO36ukFOXkCKlq~FQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 6. Actin reorganization of HVS-Normal (HVS-N8 [a through d]) and HVS-WAS (HVS-WAS1 [e through h]) T-cell lines in response to stimulation with immobilized anti-CD3 MoAb. HVS-Normal (a through d) and HVS-WAS (e through h) T cells were stimulated with immobilized OKT3 MoAb for 5 minutes (a and e), 15 minutes (b and f ), and 30 minutes (c and g). In HVS-Normal cells, actin rings are progressively dissolved, followed by cellular spreading and frequent formation of pseudopodia. In contrast, in HVS-WAS T cells, actin rings yield to strongly polarized accumulations of F-actin. HVS-WAS T cells that fail to spread acquire atypical shapes with the appearance of structures resembling uropods. Pretreatment of cells with cytochalasin D abrogates all actin-specific changes in both HVS-Normal (d) and HVS-WAS (h) immortalized T cells. (Original magnification × 400, except for [d] and [h], which are ×630.)](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/90/8/10.1182_blood.v90.8.3089/4/m_bl_0026f6f.jpeg?Expires=1769113670&Signature=3E1MYdrB-ZK48FsDk1moJe8TbD-X7Fn998hIAkPqiWwm4GRyWPgrT6AFyX7qAT0o8DQBj-2x7kbRMdTE5Zqo00rXjVT5jgQxkBr4Rt-HIk3~LdKLuVRU3Wo-hqQzPJc9beT9TLqWvfgpHntha10ZUl7QrVIQfCYenyMsolpOK~k1tjHV2bGTGF0tOv05tQLLoqQs2Kxg~l4Gs9GVbbmSBTaG6yAwkvNuHbMs0pwPH54SxV~gnsCxBPImnZm1FEDUKou0XpPiJRlSiomPGdOJiWnTt6nVhNtPHJIXokBmpp1cEi2lz8aub4TFWGtIy~icShsP5iICm6jijciYC3nxEA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 6. Actin reorganization of HVS-Normal (HVS-N8 [a through d]) and HVS-WAS (HVS-WAS1 [e through h]) T-cell lines in response to stimulation with immobilized anti-CD3 MoAb. HVS-Normal (a through d) and HVS-WAS (e through h) T cells were stimulated with immobilized OKT3 MoAb for 5 minutes (a and e), 15 minutes (b and f ), and 30 minutes (c and g). In HVS-Normal cells, actin rings are progressively dissolved, followed by cellular spreading and frequent formation of pseudopodia. In contrast, in HVS-WAS T cells, actin rings yield to strongly polarized accumulations of F-actin. HVS-WAS T cells that fail to spread acquire atypical shapes with the appearance of structures resembling uropods. Pretreatment of cells with cytochalasin D abrogates all actin-specific changes in both HVS-Normal (d) and HVS-WAS (h) immortalized T cells. (Original magnification × 400, except for [d] and [h], which are ×630.)](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/90/8/10.1182_blood.v90.8.3089/4/m_bl_0026f6g.jpeg?Expires=1769113670&Signature=Qj-Kh~n-6a5Z9EFZrCONPqtAGHn9z6F4MYu51UJgzl8zfzaac4k-HQ0GHK4qM3daA66XO7njPyZuBgRaYD8q8qv6OWdKv7SXQtUEomIUYunRR2bRPEk3ZzBzD7hVMZ6Q4Xb6-zXKvVSpBzFLTeGLX-fYri-iOkuYYSDBCHJRAoHkmPcxPjcW7wfrSZKlogyybFkO5lpDEM9L9J-1Lzgq6Ol-UqYmwuCBbxamicfIMpKDFWAn5zueeDGqDLkYDjeHuq-q-2SO01iQ4rH3AU9-3CL32lPrYRpad0I~s~AXhAgO2vnjtRTwA9oV6KuVQ14yZqj2ic56Qqva3L2pDYjlmw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 6. Actin reorganization of HVS-Normal (HVS-N8 [a through d]) and HVS-WAS (HVS-WAS1 [e through h]) T-cell lines in response to stimulation with immobilized anti-CD3 MoAb. HVS-Normal (a through d) and HVS-WAS (e through h) T cells were stimulated with immobilized OKT3 MoAb for 5 minutes (a and e), 15 minutes (b and f ), and 30 minutes (c and g). In HVS-Normal cells, actin rings are progressively dissolved, followed by cellular spreading and frequent formation of pseudopodia. In contrast, in HVS-WAS T cells, actin rings yield to strongly polarized accumulations of F-actin. HVS-WAS T cells that fail to spread acquire atypical shapes with the appearance of structures resembling uropods. Pretreatment of cells with cytochalasin D abrogates all actin-specific changes in both HVS-Normal (d) and HVS-WAS (h) immortalized T cells. (Original magnification × 400, except for [d] and [h], which are ×630.)](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/90/8/10.1182_blood.v90.8.3089/4/m_bl_0026f6h.jpeg?Expires=1769113670&Signature=Pwb60iAomhBYwA3MobmOORIDBkYicQvDTk6j3T7w0TB3PmM6hUgRvulRTr4xy8G6q-aXsK14-3JUBgyA8s7TO0noL2OQc--R9z~DKs45I1isgHpIgog51krpVmzeb6AzreyXiWMJ0RNk5wRR6vzQAWltTPF3AYK6mjbo1FYqJsLJwtpPz8W-lTiVZwkg79NmnC-3BeQGryUmtY~9QD3Dp01lVPJqQgSmulJslmdXbJLaXSnj76ktS3HeBgSPNJj8yodfuin1u99qcwjm5tRpLP8lH2JOl1bGRjhV4-GDctWySccDQFpkUu8E0jshxhrHbWBx5V1DvNfH524ZXnJGMw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal