Abstract
The underlying mechanisms of lethal cardiovascular defects associated with the fibronectin-null (FN.null) mutation in mouse embryos were investigated by lineage analysis of myocardial, endocardial, and endothelial cells. A wide variation in phenotype was observed on two genetic backgrounds. In the less severe class (C57/BL6 background), FN.null embryos display a defective heart. Myocardial cells express the specific marker MF-20 and are correctly localized in the anterior trunk region, but myocardial organization is disrupted, resulting in a bulbous heart tube. Endocardial cells express the specific marker platelet-endothelial cell adhesion molecule-1 (PECAM-1) and are localized within the myocardium, but the endocardium appears collapsed. Endothelial cells of two vascular beds are specified, but the aortae are distended and lack contact with the surrounding mesenchyme, while no vessels form in the yolk sac. Defects in the more severe class suggest that FNs are essential earlier in development on the 129/Sv background. Myocardial and endocardial cells are specified, but morphogenesis of the myocardium and endocardium does not occur. Aortic endothelial cells are specified and localized normally, but remain scattered. Yolk sac endothelial cells resemble those of the less severe class. We conclude that FNs are essential for organization of heart and blood vessels, but are dispensable for cellular specification in the appropriate regions within the embryo.
FIBRONECTINS (FNs) are major components of the extracellular matrix and blood plasma, and are specific ligands for several integrin adhesion receptors.1-3 In vitro, FNs have been shown to function in cell adhesion, migration, and extracellular matrix assembly.4,5 Wide expression of FNs in vivo suggests multiple functions in a variety of processes, including embryonic development,6-10 wound healing,11,12 and fibrosis,13 as well as immune and inflammatory responses.14-17 FNs are also overexpressed in cardiovascular disease states such as atherosclerosis18 and myocardial infarction.19 Recently, we have determined that FN expression in early development is essential, since introduction of mutations (FN.null) that disrupt expression of all FNs results in embryonic lethality.20 21
FN.null embryos display a wide variety of defects and die during gastrulation. These defects include retarded axial extension, variable absence of the primitive heart, lack of yolk sac vasculature, distortion of the neural tube, and deficiency in the mesoderm.20 However, at least two mesodermal subclasses are capable of normal cell specification, even in the absence of FNs. Pre-notochord and pre-somite cells are present in their expected locations, and express specific marker gene products. A lack of FNs prevents these cells from condensing into notochord and somites, respectively.21
While FN.null embryos display many defects, the cardiovascular abnormalities appear most likely to be the cause of lethality.22 These multiple defects culminate in an absence of blood cells within the embryo. However, hematopoiesis does not appear to be completely disrupted by a lack of FNs, since primitive red blood cells are present in the yolk sac and contain at least some hemoglobin. Instead, transport of blood cells from their site of synthesis in the yolk sac to the embryonic vasculature23,24 does not occur. This situation could arise by either an abnormality in the yolk sac vasculature or in the pumping of the heart, or both. Such defects could arise by several mechanisms, including lack of cell-type specification, abnormal localization of cells within the developing embryo, disrupted cell-cell and cell-matrix organization, reduced cellular proliferation, or inappropriate apoptosis. In vitro, FNs have been implicated in all of these processes.1 25
An additional complexity of the FN.null phenotype is that mutant embryos fall into two classes,20 apparently based on severity of the cardiovascular defects. The most variable feature is development of the primitive heart. In the more severe class, no primitive heart is observed, whereas the less severely affected embryos display a prominent and beating, albeit abnormal, primitive heart. At least two possible explanations exist for these wide variations in cardiovascular development. The first is the known variability in developmental stage within and between murine litters,24 and this could be exacerbated by the absence of FNs. Alternatively, the mixed genetic background of the FN.null mutation (129/SvXC57/BL6) may be the basis for these differences in cardiovascular development. This alternative appears likely, since strain-dependent defects have been described in mice lacking the epidermal growth factor (EGF) receptor.26 27
To investigate the underlying mechanisms of these defects in heart and blood vessel development, we have performed two types of analyses. First, we have characterized the FN.null phenotype on two pure genetic backgrounds. By determining the frequency of embryos in the two severity classes, we have shown that genetic background has profound effects on severity of the FN.null phenotype. Second, we have performed cell lineage analysis to trace cell specification, localization, and organization of cell types involved in cardiovascular development. Using specific marker gene products, we have assessed the development of myocardium, endocardium, and endothelium in FN.null mutant embryos and the yolk sac.
MATERIALS AND METHODS
Generation of FN.null mice on 129/Sv and C57/BL6 genetic backgrounds.Chimeric animals were generated essentially as previously described.28 Approximately 15 to 20 FN.null heterozygous ES cells were injected into C57/BL6 blastocysts at E 3.5. Injected embryos (10 to 12) were surgically transferred to each pseudopregnant CD1 recipient at 2.5 days postcoitum. To obtain the mutation on C57/BL6 background, chimeric males were bred to C57/BL6 females and the progeny were scored for coat color. All pups with agouti coat color were genotyped as previously described.20 Heterozygous females were further backcrossed to C57/BL6 males for one generation. In the remaining backcrosses, heterozygous males were bred to C57/BL6 females. To obtain the mutation on 129/Sv genetic background, chimeric males were bred to 129/Sv females. All progeny were genotyped as previously described.20 Heterozygous males and females were bred further to 129/Sv mice.
Whole embryo immunolabeling.Sarcomeric myosin was detected with mouse monoclonal antibody MF-20 (obtained from the Developmental Studies Hybridoma Bank, University of Iowa).29,30 Labeling of fixed E 8.0 and E 8.5 embryos from FN.null heterozygote crosses was performed by the method of Davis.31 Briefly, primary antibody was diluted in 5% bovine serum albumin in Tris-buffered saline. Embryos were exposed to primary antibody overnight at 4°C. After five 1-hour washes in Tris-buffered saline, embryos were incubated overnight with alkaline phosphatase–conjugated goat anti-mouse IgG. After five 1-hour washes in Tris-buffered saline, antibody binding was detected using nitrobluetetrazolium salt and 5-bromo-4-chloro-3-indolyl phosphate (NBT/BCIP).
Whole-mount in situ hybridization.Flk-1 mRNA was detected with a mouse cDNA clone (a generous gift from W. Risau, Bad Nauheim, Germany).32 Platelet-endothelial cell adhesion molecule-1 (PECAM-1) mRNA was detected with a mouse cDNA clone.33 Embryos were dissected from the maternal decidua and yolk sac and fixed in 4% paraformaldehyde in phosphate-buffered saline (PBS) for 2 hours. Yolk sacs were genotyped as previously described.20 Whole-mount in situ hybridization was performed as described by Conlon and Herrmann.34 Briefly, embryos were dehydrated and rehydrated in a methanol/PBS/Tween 20 series and treated with fresh proteinase K 10 μg/mL (Boehringer Mannheim Corp, Indianapolis, IN). After refixation in paraformaldehyde/glutaraldehyde, embryos were hybridized overnight at 65°C with digoxigenin-11-UTP–labeled RNA probes. After posthybridization washes and RNase A and T1 treatment, embryos were incubated with antidigoxigenin antibody coupled with alkaline phosphatase. Alkaline phosphatase was detected with NBT and BCIP as substrates. The color reaction was intensified by dehydration in a methanol series, and the embryos were cleared in glycerol.
Immunohistochemistry.PECAM-1 was detected in embryo sections with antibody MEC 13.3 (Pharmingen, San Diego, CA).35 Staged embryos were dissected from maternal decidua and fixed for 1 hour in fresh 2% paraformaldehyde/PBS on ice. After rinsing in cold PBS, embryos were equilibrated for 1 hour in OCT at room temperature. Embryos were embedded by freezing slowly over dry ice, and stored at −20°C. Cryostat sections were cut at 6 μm and melted to polylysine–treated slides. The slides were dried in air and fixed with acetone at 4°C for 5 minutes. Blocking was performed with normal goat serum/PBS followed by fetal calf serum/PBS. MEC 13.3 was used at 0.1 μg/mL at room temperature. The secondary antibody was biotinylated goat anti-rat IgG (Jackson Immuno Research, West Grove, PA) at 1:200 dilution. Biotin was detected with a Vectastain kit with standard ABC reagents and used according to the manufacturer's instructions (Vector Laboratories, Burlingame, CA), and peroxidase was detected by reaction with AEC (3′-amino, 9′ethyl′carbazole). Sections were counterstained with Gill's hematoxylin and mounted in glycerol mounting media (Sigma, St Louis, MO).
RESULTS
Genetic background affects severity of the FN.null phenotype.A notable feature of the FN.null phenotype is that mutant embryos appear to belong to two classes.20 These two classes are distinguished by the severity of the mutant phenotype, and the most notable variable is development of the primitive heart. In the more severe class, no primitive heart is detectable in whole-mount analysis. In contrast, the less severely affected embryos display a prominent primitive heart, and beating is detectable during dissection. Differences in severity also occur in the degree of shortening of the anterior-posterior axis and of reduction of headfold size. Both classes completely lack condensed somites, display a distorted neural tube, and fail to initiate the turning process.
One possible explanation for these severity differences derives from the mixed genetic background of the FN.null strain (129/SvXC57/BL6). To test this hypothesis, we compared mutant progeny on pure 129/Sv background against mutant progeny with increasing degrees of C57/BL6 background. To obtain a pure 129/Sv background, chimeric mice were generated by blastocyst injection of FN.null embryonic stem cells (129/Sv strain) as previously described.20 These chimeric males were bred with 129/Sv females, and heterozygous FN.null progeny were detected by Southern blot analysis of genomic DNA isolated from tail biopsies as previously described.20 To obtain the mutation on C57/BL6 background, male chimeras were bred with C57/BL6 females, and FN.null heterozygous progeny were bred with C57/BL6 male and female mice for six generations. To determine strain dependence of the FN.null phenotype, homozygous embryos were obtained by breeding FN.null heterozygotes at each successive backcross to C57/BL6. Homozygous embryos were examined at day 8.5 of gestation (E8.5), which is the stage at which all FN.null embryos stop progressing. All embryos from timed litters were individually dissected from maternal decidua and extra-embryonic membranes, fixed, and photographed. Embryos were examined in whole-mount preparation, and homozygotes were assigned to the less severe class if a primitive heart was visible or to the more severe class if a primitive heart was not detectable. Genotypes of all embryos were determined by Southern blot analysis of yolk sac DNA as previously described.20
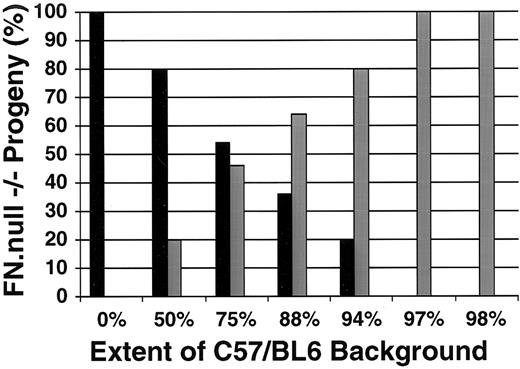
The results of this analysis are presented in Fig 1, and indicate that genetic background has a significant effect on the severity of the FN.null phenotype. On pure 129/Sv background (0% C57/BL6), we observed only mutant embryos of the more severe class; no primitive heart was visible in any mutant embryos examined (n = 6). In contrast, when heterozyous parents were both 50% C57/BL6 background, then one in five homozygous mutant embryos (n = 15) displayed a primitive heart and were assigned to the less severe class. The percentage of embryos in the less severe class increased at each successive generation of breeding to the C57/BL6 background. By the fifth generation, when the FN.null mutation was on a 97% C57/BL6 background, all homozygous mutant embryos (n = 19) were of the less severe class. This result was also observed in the sixth generation, when the mutation was on 98% C57/BL6 background. These data indicate that the FN.null mutation has a more detrimental effect on murine embryogenesis when occurring on the 129/Sv background than on the C57/BL6 background. Moreover, this strain dependence of mutant phenotype appears to be most significant in development of the primitive heart.
Genetic background affects the severity of the FN.null phenotype. The percentage of more severe (black bars) and less severe (gray bars) FN.null homozygous embryos is presented in relation to the increasing extent of strain C57/BL6 genetic background. As the extent of C57/BL6 genetic background increases, the percent of mutant embryos displaying the more severe phenotype decreases. On pure 129/Sv background (0% C57/BL6), all mutant embryos are of the more severe class, lacking overt heart development. In contrast, by the sixth breeding generation to the C57/BL6 strain (98% C57/BL6), all mutant embryos display the less severe phenotype, with a visibly detectable primitive heart.
Genetic background affects the severity of the FN.null phenotype. The percentage of more severe (black bars) and less severe (gray bars) FN.null homozygous embryos is presented in relation to the increasing extent of strain C57/BL6 genetic background. As the extent of C57/BL6 genetic background increases, the percent of mutant embryos displaying the more severe phenotype decreases. On pure 129/Sv background (0% C57/BL6), all mutant embryos are of the more severe class, lacking overt heart development. In contrast, by the sixth breeding generation to the C57/BL6 strain (98% C57/BL6), all mutant embryos display the less severe phenotype, with a visibly detectable primitive heart.
Myocardial specification and morphogenesis in the absence of FNs. FN.null embryos display wide variation in the capacity for heart development. The more severely affected embryos, which lack a primitive heart, may be defective in the specification of myocardium. In contrast, the less severe class of FN.null homozygous embryos do form a primitive heart, but our previous histologic analysis suggests that it is abnormal.20 To understand the underlying mechanisms of these defects, we assayed FN.null embryos with a specific marker of myocardial cells. A monoclonal antibody to myosin (MF-20) was used to identify myosin-positive cells in whole-mount preparations.31 This antibody has previously been used to determine the initiation and developmental distribution of myogenic precursors in rodent embryos.30 Embryos from staged FN.null heterozygous crosses were dissected from extra-embryonic membranes and fixed. Genotype was determined by polymerase chain reaction (PCR) analysis of genomic DNA isolated from the yolk sac as previously described.20 Pooled embryos were subjected to whole-mount immunohistochemistry, and bound antibody was visualized by peroxidase color reaction. These data are presented in Fig 2.
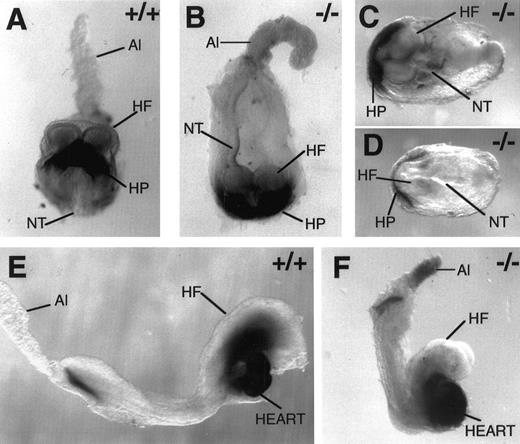
Myocardial specification, but not heart tube morphogenesis, is normal in the absence of FNs. Whole-mount immunohistochemistry with antibody MF-20 to mark myosin-positive cells in wild-type E 8.0 (A), FN.null-/- E 8.0 (B), FN.null-/- more severe class at E 8.5 (C and D), wild-type E 8.5 (E), and FN.null-/- less severe class at E 8.5 (F) mouse embryos. Note that myosin-positive cells are situated normally within the mutant embryos, even when no primitive heart is formed. In the mutant embryos in which a heart is formed, myosin-positive cells consistently display abnormal heart morphogenesis. HP, heart primordium; NT, neural tube; HF, head fold; Al, allantois.
Myocardial specification, but not heart tube morphogenesis, is normal in the absence of FNs. Whole-mount immunohistochemistry with antibody MF-20 to mark myosin-positive cells in wild-type E 8.0 (A), FN.null-/- E 8.0 (B), FN.null-/- more severe class at E 8.5 (C and D), wild-type E 8.5 (E), and FN.null-/- less severe class at E 8.5 (F) mouse embryos. Note that myosin-positive cells are situated normally within the mutant embryos, even when no primitive heart is formed. In the mutant embryos in which a heart is formed, myosin-positive cells consistently display abnormal heart morphogenesis. HP, heart primordium; NT, neural tube; HF, head fold; Al, allantois.
Comparison of wild-type and FN.null embryos at embryonic day 8.0 (Fig 2A and B) demonstrates that myocardial cell specification does not require FNs. Like normal embryos, the FN.null mutant embryo expresses myosin in cells of the crescent-shaped heart primordium. Moreover, these myosin-positive cells are present at the normal stage of embryogenesis and at the expected location within the embryo (at the base of the headfold). Also, the myosin-positive boundaries of the mutant heart primordium are approximately equal to those in normal embryos. The anterior-posterior length of the mutant embryo (Fig 2B) suggests that it represents a mutant of the less severe class. If allowed to develop until E 8.5, this embryo would likely display a primitive heart.
At E 8.5, the two classes of FN.null mutant embryos are distinctive. Embryos of the more severe class, which lack a definitive primitive heart, nevertheless express myosin-positive cells (Fig 2C and D). While the MF-20 antigen may be less abundant in these more severe embryos, it is expressed at the normal time and location compared with normal embryos. The crescent shape of the heart primordium is also maintained even in these deformed embryos. Thus, the more severe class of embryos lack a primitive heart not because of an absence of myocardial specification and/or localization. Rather, the convergence of myosin-positive cells into a primitive (straight) heart tube appears to be inhibited by a lack of FNs on this genetic background.
The less severe class of FN.null embryos, in contrast, can initiate convergence of myocardial cells. However, these embryos still display an abnormal heart. In normal mouse embryos at E 8.5 (Fig 2E), myosin-positive cells have already completed the convergence into a primitive heart tube, and are initiating the process of looping morphogenesis. In contrast, FN.null mutant embryos (Fig 2F) display a bulbous region of myosin-positive cells, which does not initiate looping. The size of this myosin-positive region varies among embryos, but it is always bulbous rather than tubular in shape. Thus, in the less severe class, heart primordial cells are specified and can also converge at the midline. However, the tubular morphology is abnormal, indicating that heart morphogenesis and/or myocardial function are disrupted at a later step. These defective hearts display beating similar to that of normal hearts at this stage.
Endocardial specification and organization in the absence of FNs.Initial histologic analysis of FN.null mutant embryos suggested abnormality in the endocardium, but was not conclusive. To assay endocardial cells definitively, we have taken advantage of the fact that these cells express PECAM-1 (also known as CD31).33 Assay of PECAM-1 in FN.null hearts allows us to determine the time of specification, localization, and organization of endocardial cells. Monoclonal antibody MEC 13.335 was used to identify PECAM-1–positive cells by immunohistochemistry of frozen sections. Embryos were genotyped by PCR analysis of embryonic DNA obtained from adjacent sections.
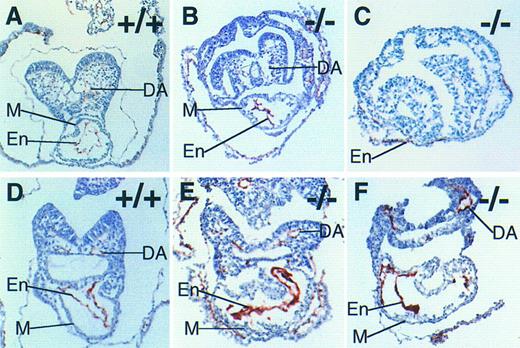
At E 8.0, the embryonic heart is a straight tube, with the endocardial layer inside the myocardial layer.24 Figure 3A shows PECAM-1–positive cells in a normal embryo, in which the endocardium displays a prominent lumen. In contrast, PECAM-1–positive cells in FN.null embryos at this stage lack a lumen (Fig 3B). The endocardium appears “collapsed.” Thus, organization of the endocardial tube is abnormal in the absence of FNs. However, the specification and localization of endocardial cells appear not to require FNs. PECAM-1–positive cells are present in FN.null mutant embryos, and are localized in the heart in normal relationship to the myocardial cell layer.
Endocardial specification, but not cellular organization, is normal in the absence of FNs. Immunohistochemistry with antibody MEC 13.3 to mark PECAM-1–positive cells in wild-type E 8.0 (A), FN.null-/- E 8.0 (B), FN.null-/- more severe class at E 8.5 (C), wild-type E 8.5 (D), and FN.null-/- less severe class at E 8.5 (E and F) mouse embryos. Note that no lumen is visible in FN.null-/- endocardium. M, myocardium; En, endocardium; DA, dorsal aorta.
Endocardial specification, but not cellular organization, is normal in the absence of FNs. Immunohistochemistry with antibody MEC 13.3 to mark PECAM-1–positive cells in wild-type E 8.0 (A), FN.null-/- E 8.0 (B), FN.null-/- more severe class at E 8.5 (C), wild-type E 8.5 (D), and FN.null-/- less severe class at E 8.5 (E and F) mouse embryos. Note that no lumen is visible in FN.null-/- endocardium. M, myocardium; En, endocardium; DA, dorsal aorta.
To determine whether endocardial cell specification can occur in the absence of heart tube formation, we assayed the more severe class FN.null mutant embryos. PECAM-1–positive cells were detected between early myocardial cells and endoderm, in a thin line that is a single cell thick (Fig 3C). This distribution of endocardial cells is similar to that in normal embryos at this stage.35 Thus, early endocardial cells and myocardial cells are in reasonably normal proximity to each other, even in the more severe class of FN.null mutant embryos. This result indicates that the absence of myocardial and endocardial apposition is not responsible for the lack of heart tube formation in the more severe FN.null mutant embryos.
To determine whether endocardial organization is simply delayed in FN.null embryos, mutant progeny were compared with normal littermates at the latest possible stage before death. At E 8.5, normal embryos have begun to loop, resulting in the straight heart tube's acquiring an S-shape. Normal endocardium is still PECAM-1–positive and is organized with a lumen (Fig 3D). However, FN.null mutant embryos possess a prominent PECAM-1–positive endocardium, but it still lacks lumen organization (Fig 3E and F). Thus, endocardial defects in FN.null embryos persist, and may be a cause of embryonic death.
Vascular patterning in the absence of FNs.Histologic analysis of FN.null mutant embryos suggested that early blood vessel development is abnormal.20 In the more severe class, the earliest blood vessels (dorsal aortae) appear to be absent. In the less severe class, structures resembling these vessels are present. However, they often appear distended and do not contain the primitive, nucleated erythrocytes that are normally produced in the yolk sac and transported into the embryo at this stage. Thus, to establish the consequences stemming from the lack of FNs on vascular development, we used two specific markers of endothelium to assay mRNA expression by whole-mount in situ hybridization. This analysis allows us to assay vascular patterning in FN.null mutant embryos. One of the earliest known markers of vascular endothelium is Flk-1, a receptor tyrosine kinase, which is one of two known transmembrane receptors for vascular endothelial growth factor (also known as vascular permeability factor).32 PECAM-1 mRNA is also expressed in early vascular endothelium, and expression persists at later stages in development.33 Progeny of heterozygous FN.null matings were dissected from extra-embryonic membranes, fixed, and hybridized with digoxigenin-labeled antisense riboprobes. Hybridized probe was detected by the color reaction product of phosphatase-conjugated antidigoxigenin. All embryos were genotyped by PCR analysis of yolk sac DNA.
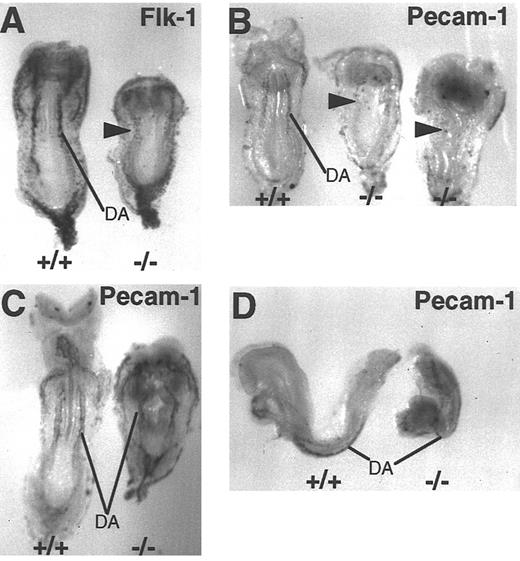
The apparent absence of dorsal aortae in more severe FN.null mutant embryos suggests that endothelial cell precursors may not be specified. Alternatively, endothelial cells may be specified but incapable of blood vessel morphogenesis. To distinguish between these possibilities, more severe FN.null embryos were probed for both Flk-1 and PECAM-1. The Flk-1 expression pattern in mutant embryos demonstrates that endothelial precursors are present and in the normal location lateral and ventral to the neural tube (Fig 4A). However, compared with the wild-type littermate, Flk-1–positive FN.null cells are less abundant and more widely dispersed. In contrast, these more severe mutant embryos contain few PECAM-1–positive cells (Fig 4B). Two examples of the more severe class are shown, and both display only scattered PECAM-1–positive cells. Compared with the wild-type littermate at the left of the figure, none of the positive cells display normal patterning of the dorsal aortae. These results are consistent with the presence of specified Flk-1–positive endothelial cells, only a few of which also express PECAM-1.
Vascular patterning is detected in FN.null embryos even in the absence of organized blood vessels. Whole-mount in situ hybridization with antisense Flk-1 probe to mark early endothelium in wild-type and more severe class FN.null-/- at E 8.5 (A). Hybridization with antisense PECAM-1 probe to mark early endothelium in wild-type and 2 more severe class FN.null-/- at E 8.5 (B) and wild-type and less severe class FN.null-/- at E 8.5 (C and D). Note that in the more severe class, only the pattern of dorsal aortae is visible in Flk-1–positive cells. In the less severe class, PECAM-1–positive cells are organized into blood vessels. DA, dorsal aortae. Arrowheads indicate endothelial cells.
Vascular patterning is detected in FN.null embryos even in the absence of organized blood vessels. Whole-mount in situ hybridization with antisense Flk-1 probe to mark early endothelium in wild-type and more severe class FN.null-/- at E 8.5 (A). Hybridization with antisense PECAM-1 probe to mark early endothelium in wild-type and 2 more severe class FN.null-/- at E 8.5 (B) and wild-type and less severe class FN.null-/- at E 8.5 (C and D). Note that in the more severe class, only the pattern of dorsal aortae is visible in Flk-1–positive cells. In the less severe class, PECAM-1–positive cells are organized into blood vessels. DA, dorsal aortae. Arrowheads indicate endothelial cells.
In contrast, less severe FN.null mutant embryos have close to the normal number of PECAM-1–positive cells compared with wild-type littermates (Fig 4C and D). Moreover, PECAM-1–positive endothelial cells are patterned normally as paired dorsal aortae on either side of the neural tube. Mutant dorsal aortae maintain a parallel relationship with the mutant neural tube; the vessels bend in the same pattern as the neural tube kinks (Fig 4C). Less severe mutant embryos also express Flk-1 mRNA in a normal pattern (data not shown).
FN.null embryos display distinct defects between intra- and extra-embryonic vasculogenesis.The whole-mount identification of vascular endothelium shown in Fig 4 demonstrates the ability of embryos to establish the vascular pattern in the absence of FNs. However, organization of endothelial cells into blood vessels is not resolvable at the level of the whole-mount embryo. Therefore, to assay vasculogenesis at the cellular level, we performed immunohistochemistry on sectioned FN.null mutant embryos. Distribution of PECAM-1 protein was compared in normal and mutant littermates in both embryonic and extra-embryonic vasculogenesis. All embryos were genotyped by PCR analysis of DNA extracted from adjacent sections.
The extra-embryonic yolk sac is the first site of hematopoiesis in the mouse embryo, and upon fusion of the yolk sac and embryonic vasculatures, it is the primary source of blood cells for the embryo.23,24 Composed of mesodermal and visceral endodermal cell layers, the yolk sac mesodermal cells proliferate and differentiate into blood islands. Individual blood islands fuse by vasculogenesis, forming a vascular network containing primitive, nucleated red blood cells. Previous histologic analysis of FN.null yolk sac revealed abnormal separation of the two cell layers,20 but endothelial cells were not assayed. In normal yolk sac at E 8.5, PECAM-1–positive endothelial cells line the newly formed blood vessels (Fig 5A). However, in FN.null yolk sac, the organization of PECAM-1–positive cells is abnormal. The FN.null yolk sac mesoderm does express PECAM-1, but likely at reduced levels (Fig 5B). Moreover, the PECAM-1–positive cells are not distributed as a lining of blood vessels, but are only associated with the mesodermal layer. Whether this is a consequence of separation of the cell layers or whether it contributes to the separation is not known.
Embryonic and extra-embryonic vasculogenesis is affected differently by a lack of FNs. Immunohistochemistry with antibody MEC 13.3 to mark PECAM-1–positive endothelial cells in wild-type (A) and FN.null-/- less severe (B) yolk sac and wild-type (C) and FN.null-/- less severe (D) dorsal aortae. In the mutant yolk sac, PECAM-1–positive cells are associated with the mesodermal layer only. In the wild-type yolk sac, endothelium lines the blood vessel. In contrast, PECAM-1–positive endothelium lines both wild-type and FN.null-/- dorsal aortae. Within the embryo, a lack of FN does not prevent organization of the blood vessel, but rather its maintenance. E, yolk sac endoderm; M, yolk sac mesosderm; BC, primitive blood cells; DA, dorsal aortae.
Embryonic and extra-embryonic vasculogenesis is affected differently by a lack of FNs. Immunohistochemistry with antibody MEC 13.3 to mark PECAM-1–positive endothelial cells in wild-type (A) and FN.null-/- less severe (B) yolk sac and wild-type (C) and FN.null-/- less severe (D) dorsal aortae. In the mutant yolk sac, PECAM-1–positive cells are associated with the mesodermal layer only. In the wild-type yolk sac, endothelium lines the blood vessel. In contrast, PECAM-1–positive endothelium lines both wild-type and FN.null-/- dorsal aortae. Within the embryo, a lack of FN does not prevent organization of the blood vessel, but rather its maintenance. E, yolk sac endoderm; M, yolk sac mesosderm; BC, primitive blood cells; DA, dorsal aortae.
Vasculogenesis also occurs within the embryo at this stage, forming the dorsal aortae.24 In normal embryos, PECAM-1–positive cells line these vessels and maintain tight contact with the surrounding mesoderm (Fig 5C). In contrast, PECAM-1–positive cells in FN.null mutant embryos are separated from the surrounding mesoderm (Fig 5D). However, the continuous ring of PECAM-1–positive cells indicates that endothelial organization into a vessel has occurred. Thus, embryonic vasculogenesis appears to require FNs only for attachment and/or maintenance of the endothelium in contact with the mesoderm, not for organization of the endothelial tube. This result is in contrast to our findings with yolk sac vasculogenesis, in which blood vessels do not form in the absence of FNs.
DISCUSSION
Defects in heart and blood vessel development in FN.null embryos are likely the primary cause of their death in utero.22 To determine the underlying mechanisms responsible for these defects, we performed cell lineage analyses and investigated the influence of genetic background on the mutant phenotype. Our data indicate that genetic background has a profound effect on the severity of the FN.null phenotype, resulting in two distinct classes of mutant embryos depending on background strain. On a C57/BL6 background, FN.null embryos display a defective, primitive heart that is bulbous rather than tubular in shape. However, on a 129/Sv background, no primitive heart is formed. Lineage analysis of both phenotypic classes demonstrates that precursor cells of the myocardium are specified and positioned normally. Thus, we conclude that FNs are essential components during morphogenesis of the heart tube, but are not required for specification or embryonic localization of myocardial precursor cells. Similarly, lineage analysis of the FN.null endocardium demonstrates that FNs are essential for formation and/or maintenance of the lumen, but are dispensable for specification of endocardial precursor cells and their localization. In the less severe class, endocardial cells are specified and positioned normally within the myocardial tube, but lack a lumen and appear collapsed. In the more severe class lacking a heart tube, endocardial cells are still specified and positioned normally in the embryo.
In mutant embryos with a primitive heart (C57/BL6 background), both myocardial and endocardial cells are defective in organization. Either or both of these cell types may be directly affected by the FN.null mutation, or one cell type may affect the other. Communication between the endocardium and myocardium has been demonstrated in adults,36 and recently also in zebrafish embryos.37 The cloche mutation results in an absence of endocardium, which reduces contractility in the myocardium. This intercellular communication could be disrupted if the primary FN.null heart defect is in the complex extracellular matrix that is localized between these cell layers, known as the cardiac jelly. In rodent embryos, FNs are normally synthesized by the endocardial cells and are abundant components of the cardiac jelly,38 as is the glycosaminoglycan hyaluronic acid.39 A lack of FNs in the cardiac jelly could disrupt matrix assembly. At least one other extracellular matrix protein, fibulin, is known to depend on FNs for assembly.40 Fibulin is present in the myocardial basement membrane, surrounds endocardial cells, and extends into the cardiac jelly in the developing chick heart, but its expression in mouse embryos is not known.41 Temporal expression of FN mRNA and protein during rat embryogenesis supports the idea that the cardiac jelly is the first essential site of FN deposition in the heart.38 Early, when the myocardium is folding toward the midline, FN mRNA is significantly downregulated in the surrounding mesoderm. Only later, at the straight heart tube stage, is FN synthesized at high levels. This FN is synthesized by the endocardial cells, and is thought to be the sole source of FN in the cardiac jelly.38 Slightly later in development, just before cardiac cushion tissue formation, FNs appear to complex with ES proteins, and the resulting particles are known as adherons.42 Unfortunately, FN.null embryos do not progress far enough in development to allow us to determine if FNs are essential during this epithelial-mesenchymal transition in the formation of cardiac cushions.
In contrast, in the more severe mutant class (129/Sv background), myocardial cells do not form a tube at the midline. This suggests that FNs are essential much earlier, during formation of the heart tube. Thus, in contrast to the C57/BL6 background, a lack of FNs on the 129/Sv background disrupts myocardial migration and/or apposition of the lateral body folds. Such wide variability in mutant phenotype on different genetic backgrounds has also been observed in mice with a null mutation in the EGF receptor.26,27 Wide variation in heart defects is also observed clinically in patients with congenital heart disease; the same genetic abnormality affects some patients more severely than others.43 We have also shown that the frequency of the two mutant classes depends on the degree of backcross. This progression of change at each generation suggests that two or three genes, which differ between these genetic backgrounds, interact with the FN.null mutation acting either to suppress or to enhance its effects. Mapping and identification of the interacting genes responsible for this variability are required for further conclusions. Two candidate classes of genes that might have an impact on the severity of the FN.null phenotype are those encoding other matrix molecules and/or adhesion receptors.
The defects that we observe in vasculogenesis in FN.null embryos demonstrate that FNs are essential in this process. Alternative in vivo experiments, using specific antibodies to either β1- or αvβ3-integrin extracellular matrix receptors, also result in abnormal vasculogenesis in the dorsal aortae.44 45 Thus, a disruption or lack of either receptor or ligand (FN is a known ligand, although not a major one for αvβ3, for both of these integrin receptors) can have profound effects on blood vessel development. These defects in vasculogenesis are also influenced by genetic background. On the 129/Sv background, endothelial cell precursors are specified in at least some of the expected locations, but do not coalesce into vessels. In contrast, some vessels are formed on the C57/BL6 background, but are abnormal. Also, a lack of FNs has distinct effects depending on the vascular bed. In dorsal aortae, vessels are dilated, and contact between the endothelium and the surrounding mesoderm is not maintained. However, the specification and patterning of aortic endothelial cells and lumen formation appear normal in the absence of FNs on this background. In contrast, extra-embryonic (yolk sac) vasculogenesis is more severely disrupted in the absence of FNs. On both genetic backgrounds, endothelial cells are specified in the yolk sac, but do not become organized into endothelial tubes. Instead, yolk sac mesoderm and endoderm cell layers dissociate in the absence of FNs.
The distinct defects observed in FN.null intra- versus extra-embryonic vascular development support the idea that these processes have distinct underlying mechanisms.46,47 In the yolk sac, endothelial differentiation occurs concomitantly with hematopoietic precursors in the blood islands. In contrast, most intra-embryonic endothelium differentiates in the absence of hematopoiesis. Our data establish distinct requirements of the extracellular matrix in these related processes. In the yolk sac, an absence of FN results in separation of mesodermal and endodermal cell layers, suggesting that contact between these two cell layers is required for vasculogenesis but not for specification of endothelium. This conclusion is supported by yolk sac explant experiments in which separation of mesoderm and endoderm results in an absence of a vascular network.48 However, similar to the FN.null yolk sac, endothelial markers were expressed relatively normally in the separate cell layers. Also, vascular development is disrupted in GATA-4 -/- embryoid bodies, which lack yolk sac endoderm.49 The endoderm cell layer appears to be the source of basic FGF required for vasculogenesis in the yolk sac, and reduction of basic FGF there by maternal exposure to retinoic acid results in an avascular yolk sac.50 Whether the yolk sac defects resulting from a lack of FNs are due to disrupted cell–extracellular matrix adhesion or to defects in cell-cell signaling remains to be determined. In the normal mouse yolk sac, FNs are synthesized by mesodermal cells38 and assemble into matrix between the two cell layers.20 This distribution of FNs is consistent with a lack of cell adhesion as the explanation for the dissociated cell layers. An alternative explanation is also possible, and FNs may be involved in outside-in signaling via ligation of integrin receptors on the cell surface.2 Recently, dissociation of yolk sac cell layers has been shown to be caused by defects other than simple cell–extracellular matrix adhesion. G-protein subunit-α13 -/- embryos also display a defective yolk sac in which the cell layers are separated.51 Further experiments are necessary to determine if these interesting results affect each other.
FN.null embryos are viable only long enough to observe vasculogenesis of the dorsal aortae. On both genetic backgrounds, endothelial precursor cells are positioned normally along the anterior-posterior axis. However, only on the C57/BL6 background do these cells form vessels with a lumen. Lineage analysis demonstrates that FN.null endothelial cells form dilated vessels, and fail either to establish or to maintain contact with the surrounding mesenchyme. At this stage of mouse development, FNs are abundant in the mesenchyme during formation of endothelial tubes, and persist in the basement membrane surrounding blood vessels.20 This expression pattern is consistent with the idea that cell–extracellular matrix adhesion is the underlying cause of endothelial dilation and lack of mesenchymal contact. However, recent data on involvement of the surrounding mesenchyme in maturation and stabilization of blood vessels may also be of significance in this question. The recently discovered TIE2 receptor ligand angiopoietin appears to be required for endothelial cells to recruit surrounding mesenchyme to the newly formed tube during blood vessel formation.52 53 The fact that FN.null endothelial tubes do form but lack contact with the surrounding mesenchyme is reminiscent of this model. Thus, FNs may also be involved in signaling, either by the TIE2/angiopoietin pathway or by some other system. FNs may facilitate transport of intercellular signaling molecules, or may be required for migration of mesenchymal cells. Further analysis is necessary to address these intriguing possibilities.
ACKNOWLEDGMENTS
We are grateful to Steven Robinson for helping with some of the backcrossing, Hong Tan for assistance in obtaining staged embryos, and Sharon Kim and George Stavrakis for assistance in immunohistochemistry. E.L.G. was a Special Fellow of the Leukemia Society of America. H.S.B. is an Established Investigator of the American Heart Association. R.O.H. is a Howard Hughes Medical Institute Investigator.
Supported by National Institutes of Health Grants No. GM 52366 (E.L.G.), HL 56583, HL 51533, DCD 02027 (H.S.B.), and PO1HL 41484 (R.O.H.), American Heart Association Grant No. 95014210 (H.S.B.), a grant from the M.A. Smith Lead Charitable Trust (H.S.B.), and the Howard Hughes Medical Institute.
Address reprint requests to Elizabeth L. George, PhD, Vascular Research Division, Department of Pathology, Brigham and Women's Hospital and Harvard Medical School, Boston, MA 02115.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal