Abstract
Lung epithelial cells (A549) synthesize and secrete fibrinogen (FBG) in vitro when stimulated with interleukin-6 and dexamethasone. This FBG secretion is polarized in the basolateral direction, suggesting that FBG is a component of the extracellular matrix (ECM). Immunofluorescent staining of A549 cells showed a fibrillar pattern of FBG, similar to the staining detected using antibodies against the matrix constituents, collagen type IV and fibronectin (FN). The same pattern of staining was detected using antibodies against fibrinopeptides A and B, as well as with the T2G1 monoclonal antibody against the fibrin-specific epitope, β15-21. Matrix staining was unaltered in the presence of the thrombin inhibitor, hirudin, or the plasmin inhibitor, aprotinin, consistent with the interpretation that matrix deposition of FBG does not require such enzymatic action. Metabolic labeling studies confirmed that FBG secreted from A549 cells or deposited into the ECM showed no evidence of thrombin or plasmin proteolytic processing or of transglutaminase-mediated covalent cross-linking (γ-γ dimers or α-polymers). Incubation of either A549 cell-derived or purified plasma FBG with cultures of human foreskin fibroblasts resulted in FBG deposition in the ECM that colocalized with matrix fibrils containing endogenously produced FN and laminin (LN). Binding of FBG to this exogenously produced matrix was unaltered by inhibition of thrombin and plasmin action, yet also exhibited exposure of the fibrin-specific epitope, β15-21. The majority (∼70%) of newly synthesized and secreted FBG is bound to the cell surface as determined by its trypsin-sensitivity. Cell surface-bound FBG is initially deoxycholate-soluble, which, over time, becomes incorporated in the deoxycholate-insoluble ECM in a similar fashion to FN. These data suggest that matrix incorporation requires the binding of secreted FBG to cell-associated matrix assembly sites. However, unlike FN, FBG in the ECM is composed of the dimeric protamer (Aα/Bβ/γγ) and not high molecular weight polymers indicative of fibrin. This study provides evidence that deposition of FBG in both endogenous and exogenously produced matrices results in conformational changes that occur independently of thrombin cleavage. This matrix-bound FBG, on which unique cell-reactive domains are likely exposed, could augment cellular response mechanisms evoked during injury and inflammation.
TISSUE INJURY INDUCES an inflammatory response that results in upregulation of hepatic fibrinogen (FBG) synthesis and its secretion along with other acute-phase reactants.1 Cutaneous or vascular injury activates blood coagulation, resulting in thrombin-mediated conversion of FBG to fibrin via the enzymatic release of fibrinopeptides A and B (FPA and FPB).2-4 The resulting fibrin provisional matrix facilitates platelet adherence, angiogenesis, cell migration, and cell spreading.5-10 Although the liver is the primary source of plasma FBG, several studies have shown a basal, constitutive expression of the FBG γ chain in extrahepatic tissues.11-13 Epithelial cells from intestine and cervix have been shown to synthesize fully assembled FBG in vitro.14,15 Cultured lung epithelial cells (A549) synthesize and secrete FBG after stimulation with interleukin-6 (IL-6) and dexamethasone (DEX),16 factors present during the acute-phase response.3 Furthermore, when A549 cells are cultured on polycarbonate filters, FBG secretion is polarized, with greater than 80% directed basolaterally.17 These findings suggest that FBG may be targeted to the basement membrane when synthesized in vivo.
Several proteins, including fibronectin (FN), thrombospondin, vitronectin, von Willebrand factor, and fibulin,18-23 function in both plasma and as components of basement membranes. Whereas plasma FBG has been well-characterized for its role in hemostasis, FBG has not heretofore been recognized as a component of extracellular matrices. This study examines the extracellular destination and structure of FBG. The results indicate that (1) nascent secreted FBG binds to the cell surface, (2) lung epithelial cells incorporate newly synthesized FBG into the extracellular matrix (ECM), (3) secreted FBG binds to exogenously produced matrix, and (4) upon its deposition in the matrix, FBG displays an epitope characteristic of fibrin. In that there is no evidence of thrombin or plasmin enzymatic action, it appears that surface-bound FBG undergoes a conformational change exposing a fibrin-like epitope. Furthermore, these results suggest that matrix-bound FBG may influence cell behavior in the context of wound repair and inflammation.
MATERIALS AND METHODS
Cells and culture conditions.A549 human lung epithelial cells (CCL 185) and human foreskin fibroblasts (HFF )24 were grown to confluent monolayers in Kaighn's Nutrient Mixture F12 medium (Irvine Scientific Co, Santa Ana, CA) with 10% fetal bovine serum (FBS; Intergen, Purchase, NY).16 All cell culture reagents were obtained from Life Technologies (Gaithersburg, MD). A549 and HFF were seeded onto T-25 cm2 flasks, 6-well plates (Costar, Cambridge, MA), or 3.0-mm glass coverslips (Electron Microscopy Sciences, Fort Washington, PA). For induction of FBG synthesis, cells were stimulated with IL-6 (25 U/mL) and DEX (0.1 μmol/L).16 For studies involving thrombin or plasmin inhibition, hirudin (1 U/mL; Sigma, St Louis, MO) and aprotinin (200 KIU/mL; Miles, Kankakee, IL), respectively, were added to culture media. Human thrombin (CalBiochem, La Jolla, Ca) was used at 1.0 U/mL and plasmin was used at at 0.25 U/mL.
Immunofluorescent staining.Immunofluorescent labeling of cells cultured on glass coverslips was performed.17 After treatment with DEX + IL-6, cells were washed twice with 1 mL phosphate-buffered saline, fixed in 3.7% formaldehyde, permeabilized with 0.5% Triton X-100 when required, and stained with 10 μg/mL primary antibody as listed in Table 1. Monoclonal antibody (MoAb) raised against chicken α-tubulin, which also recognizes human α-tubulin, was obtained from Amersham (Arlington Heights, IL). Rhodamine- and fluorescein-conjugated antibodies to rabbit or mouse Igs (Cappel, West Chester, PA) were used as secondary antibodies.
Iodination, metabolic labeling, and immunoprecipitation.Purified human FBG (CalBiochem) was depleted of FN by gelatin-Sepharose chromatography and radioiodinated.17 For studies analyzing 125I-FBG deposition in matrix, 2, 10, or 50 μg/mL of 125I-labeled FBG was added to HFF cultured in T-25 cm2 flasks and incubated at 37°C for 72 hours. The washed cell monolayers were fixed and then stained by indirect immunofluorescence as described above. For controlled experiments analyzing thrombin activity, 10 μg/mL 125I-FBG was used per 1 mL of reaction. Metabolic labeling of cellular proteins was performed with 35S-cys + 35S-met Express Protein Labeling Mix (Dupont-NEN, Boston, MA). Unless otherwise specified, cells were lysed in standard cell lysing buffer consisting of 0.5% deoxycholate (DOC), 0.5% nonidet P-40, 0.1% sodium dodecyl sulfate (SDS), 1 mmol/L N-ethylmaleimide (NEM), 1 mmol/L iodoacetic acid (IAA), 2 mmol/L phenylmethylsulfonyl fluoride (PMSF ), 2 mmol/L EDTA in 0.1 mol/L Tris-HCl, pH 8.3. Labeled FBG was immunoprecipitated from culture media or cell lysate using purified rabbit antihuman FBG IgG coupled to protein A-Sepharose (Sigma).16 After immunoprecipitation, samples were analyzed by electrophoresis on Laemmli or Weber Osborn polyacrylamide-SDS gels30 or 2% agarose-SDS gels.16
Analysis of cell/matrix-associated FBG.To determine whether cell/matrix-associated FBG is intracellular, bound to cells, and/or incorporated into the ECM, confluent cultures of A549 cells were induced for 18 hours with DEX + IL-6 and then metabolically labeled (100 μCi/mL) for up to 120 minutes. At the end of each time point, media were saved and the cells were washed twice with 1 mL Hank's Balanced Salt Solution. Control cells were scraped and lysed immediately in standard lysing buffer; trypsinized cells were lifted with 1 mL trypsin:EDTA (0.05%:0.53 mmol/L) by incubation for 5 minutes at 37°C. Trypsin action was stopped with soybean trypsin inhibitor at 500 μg/mL.31
Analysis of detergent-insoluble FBG.To determine whether cell/matrix-associated FBG is a component of the DOC-insoluble fraction of the A549 ECM, cells were treated with DEX + IL-6 for 18 hours, pulse-labeled with 1 mCi/mL 35S-cys + met for 10 minutes, and chased with unlabeled complete medium for 2, 6, and 24 hours. At the end of the chase period, the culture medium was removed and the cell monolayers were washed three times in phosphate-buffered saline. The cells were scraped and lysed in 2% DOC in 20 mmol/L Tris-HCl, pH 8.8, containing 2 mmol/L each of PMSF, EDTA, IAA, and NEM. The DOC-insoluble fraction was recovered by centrifugation, and the pellet was washed and then solubilized in 1% SDS in 20 mmol/L Tris-HCl, pH 8.8, with 2 mmol/L each of PMSF, EDTA, IAA, and NEM.31 32 FBG was immunopurified and analyzed by SDS-polyacrylamide gel electrophoresis (SDS-PAGE) after reduction on 6% polyacrylamide gels or without reduction on 2% agarose gels. Scanning densitometry was performed using the NIH Image 1.59 software and a ScanJet IIcx (Hewlitt Packard, Palo Alto, CA).
RESULTS
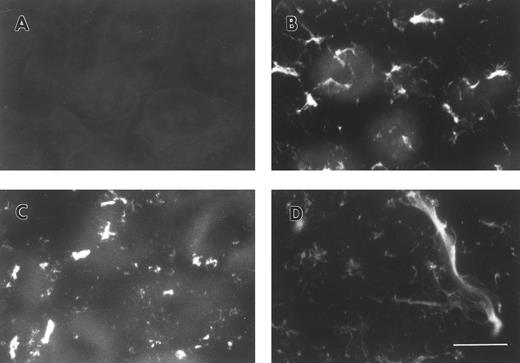
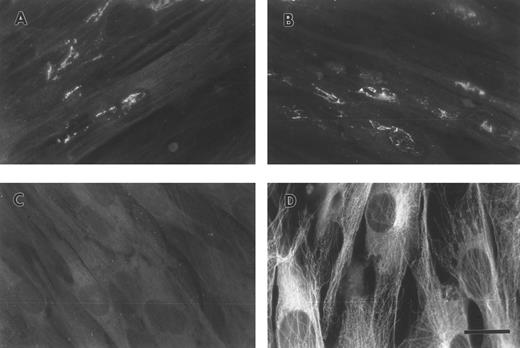
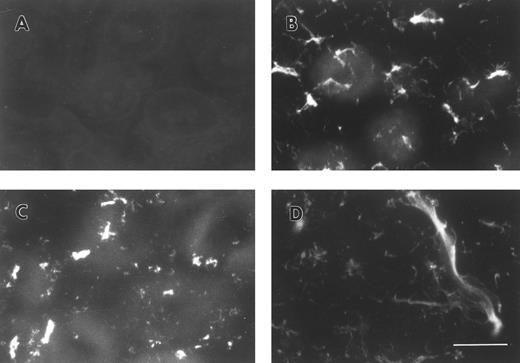
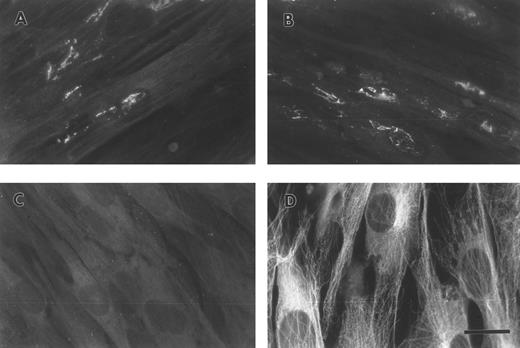
FBG synthesized by A549 lung epithelial cells is deposited into the ECM.A549 cells were cultured to confluence on glass coverslips and then treated for 18 hours with IL-6 + DEX to induce FBG expression. Immunofluorescent staining was then performed using antibodies specific for the matrix constituents FN and collagen type IV, in parallel with an MoAb that recognizes fibrin(ogen) γ chain residues 63 through 78.25 Immunostaining for FN and collagen type IV on both untreated (not shown) and IL-6 + DEX–treated cells showed the presence of an extensive fibrillar ECM beneath and between cells (Fig 1C and 1D). Immunostaining using the FBG-specific MoAb J88B resulted in the appearance of a similar fibrillar pattern, which was present in IL-6 + DEX–treated cells (Fig 1B), but absent in unstimulated cells (Fig 1A). This result is consistent with the prior observation that unstimulated A549 cells express negligible quantities of FBG.16
Immunofluorescent detection of FBG in lung epithelial cell ECM. A549 cells grown on glass coverslips were treated with IL-6 and DEX for 18 hours (B through D) or untreated (A), fixed, and then stained with the following antibodies: (A and B) MoAb J88B for FBG; (C) PoAb for FN; and (D) MoAb COL-94 for collagen type IV. The bar in (D) represents 10 μm.
Immunofluorescent detection of FBG in lung epithelial cell ECM. A549 cells grown on glass coverslips were treated with IL-6 and DEX for 18 hours (B through D) or untreated (A), fixed, and then stained with the following antibodies: (A and B) MoAb J88B for FBG; (C) PoAb for FN; and (D) MoAb COL-94 for collagen type IV. The bar in (D) represents 10 μm.
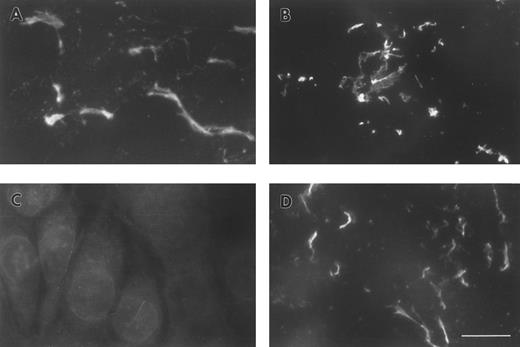
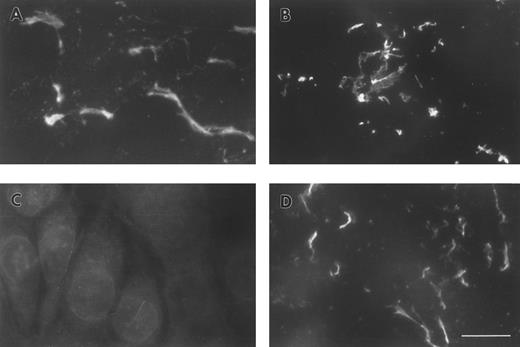
FBG deposition in the ECM results in exposure of fibrin-specific epitopes in the absence of thrombin cleavage, γ-γ dimer formation, and plasmin degradation.A549 cell matrix-bound FBG was immunostained with those antibodies recognizing fibrinopeptides that are cleaved during FBG conversion to fibrin (Table 1). Both FPA and FPB were present in A549-matrix FBG, as shown by the intensely stained fibrillar pattern detected using antibodies against FPA (RDV3; Fig 2A) and FPB (18C6; Fig 2B). These staining patterns were similar to those detected using the fibrin (ogen)-specific MoAb J88B (Fig 1B). To determine whether fibrin-specific epitopes are exposed on matrix-bound FBG as well, immunostaining was conducted using MoAb T2G1, which recognizes the fibrin-specific epitope β15-21 (Table 1). A comparable staining pattern was shown (Fig 2D), suggesting exposure of this fibrin-specific epitope.
ECM of A549 lung epithelial cells stained for FBG and fibrin-specific epitopes. IL-6 + DEX–treated cells were stained with (A) MoAb RDV3 for FPA; (B) MoAb 18C6 for FPB; (C) no primary antibody; or (D) MoAb T2G1 for fibrin β15-21. The bar in (D) represents 10 μm.
ECM of A549 lung epithelial cells stained for FBG and fibrin-specific epitopes. IL-6 + DEX–treated cells were stained with (A) MoAb RDV3 for FPA; (B) MoAb 18C6 for FPB; (C) no primary antibody; or (D) MoAb T2G1 for fibrin β15-21. The bar in (D) represents 10 μm.
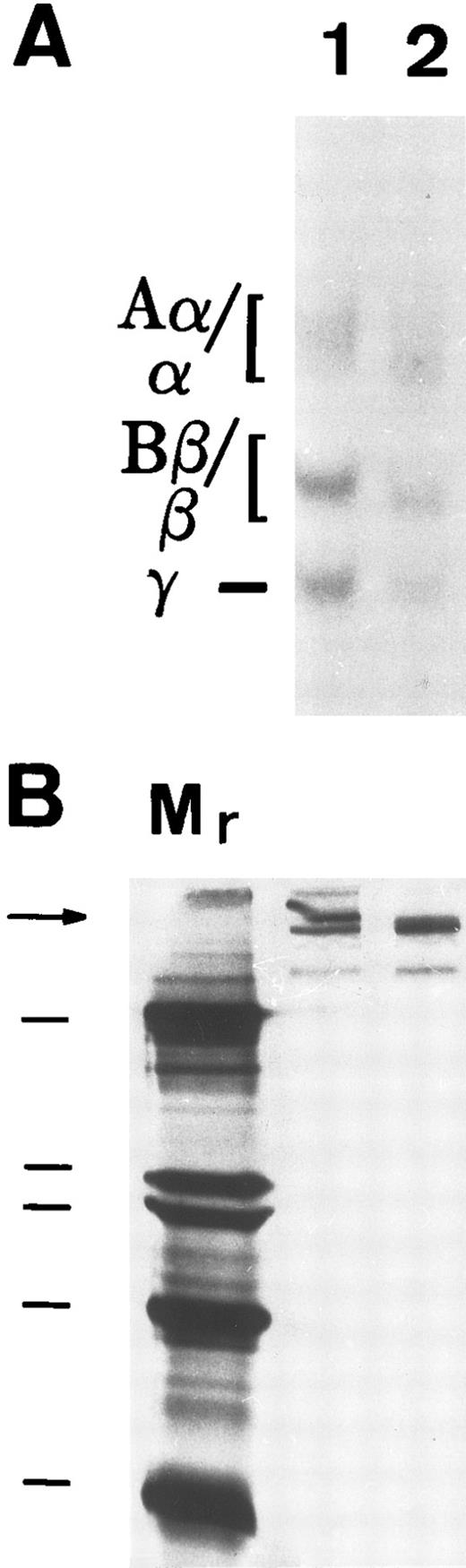
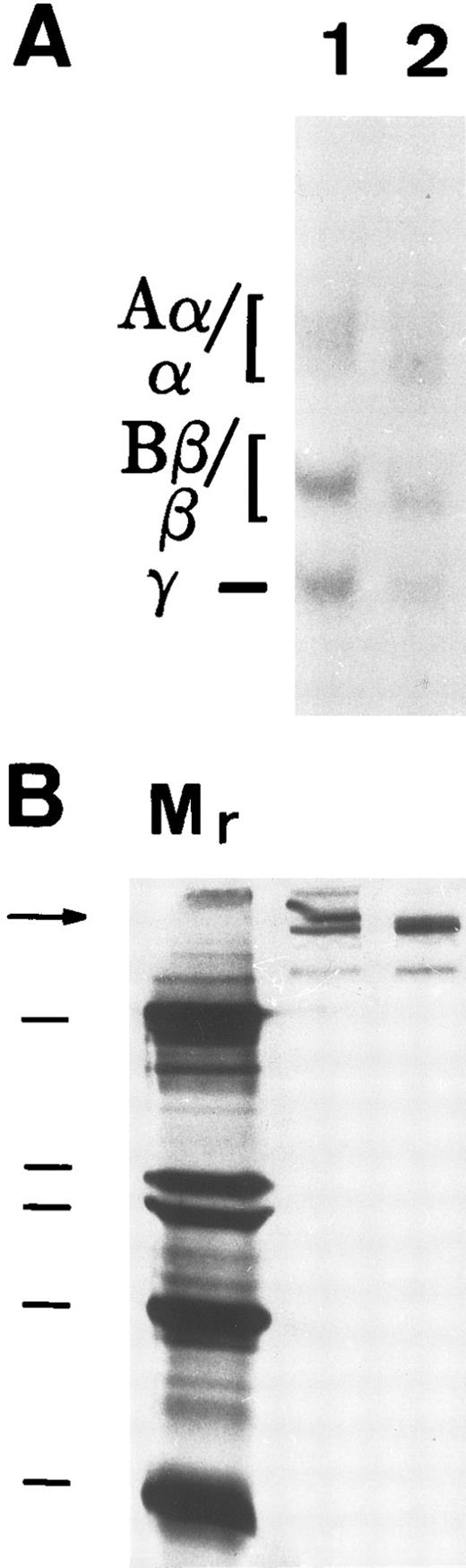
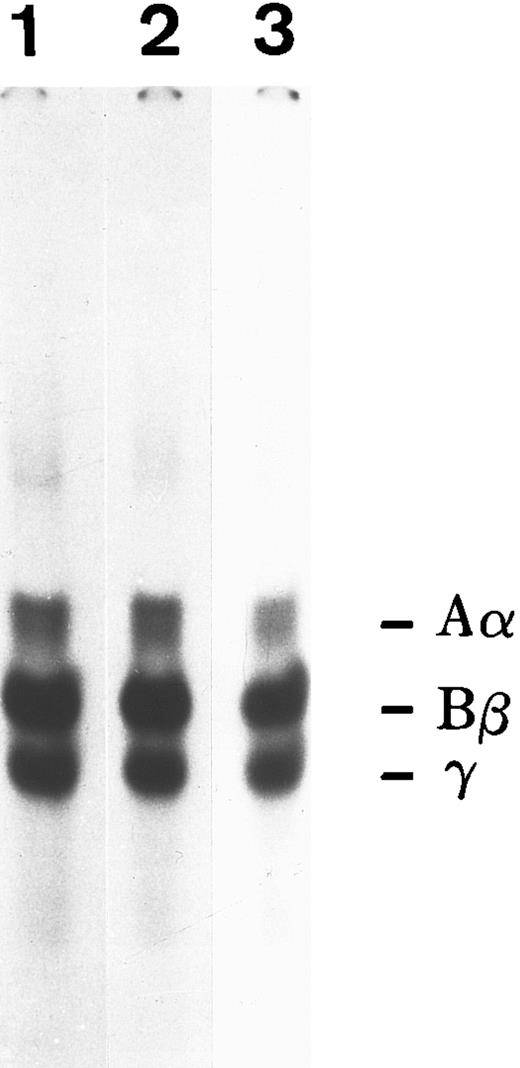
To determine if matrix incorporation necessitated enzymatic cleavage of FBG, A549 cells were cultured in the presence of hirudin and aprotinin. The resultant matrix staining pattern was unaffected by the presence of these inhibitors; thus, FPA, FPB, and β15-21 epitopes are exposed (Table 2). To further confirm lack of thrombin or plasmin action on A549 cell-derived FBG, cells were metabolically labeled during the 18 hours of IL-6 + DEX induction and FBG was immunopurified from culture medium and analyzed by SDS-PAGE, followed by fluorography. FBG secreted from A549 cells showed no evidence of cleavage by thrombin (Fig 3A, lane 1) or plasmin (Fig 3B, lane 1). Furthermore, there was no evidence of the formation of γ-γ dimers or high molecular weight α-polymers indicative of enzymatic cross-linking and insoluble polymer formation (Fig 3A). Subsequent exposure of these samples to thrombin resulted in the expected shift in migration of both the α and β chains (Fig 3A, lane 2), demonstrating that lung epithelial cell-derived FBG is sensitive to thrombin cleavage under these experimental conditions. In contrast, FBG from A549 cell-conditioned media incubated for 18 hours with plasmin did not undergo cleavage, because characteristic degradation products were absent (Fig 3B, lane 2). These results suggest that, whereas thrombin cleavage of FBG can occur under the culture conditions used, plasmin cleavage is not likely to occur due to the presence of FBS-derived plasmin inhibitors. There was no evidence of thrombin or plasmin action on 125I-labeled purified plasma FBG when incubated with 10% FBS containing medium (Fig 4C). However, 125I-plasma FBG (10 μg/mL) incubated in serum-free medium was susceptible to cleavage by these enzymes, because the addition of plasmin (Fig 4A, lane 2) or thrombin (Fig 4B, lane 2) resulted in the appearance of the expected cleavage products.
Susceptibility of A549 lung cell-derived FBG to thrombin and plasmin cleavage. (A) Metabolically labeled FBG in A549 conditioned media was untreated (lane 1) or treated with thrombin (lane 2) and immunopurified and the resulting fibrin(ogen) Aα/α, Bβ/β, and γ chains were reduced then resolved on 7% polyacrylamide gels. (B) The untreated (lane 1) and plasmin-treated (lane 2) samples were resolved nonreduced on 8% polyacrylamide gels. The molecular weight markers (lane Mr ) in (B) are, from top to bottom, 200, 97, 68, and 46 kD. Intact FBG (340 kD) is denoted by the arrow.
Susceptibility of A549 lung cell-derived FBG to thrombin and plasmin cleavage. (A) Metabolically labeled FBG in A549 conditioned media was untreated (lane 1) or treated with thrombin (lane 2) and immunopurified and the resulting fibrin(ogen) Aα/α, Bβ/β, and γ chains were reduced then resolved on 7% polyacrylamide gels. (B) The untreated (lane 1) and plasmin-treated (lane 2) samples were resolved nonreduced on 8% polyacrylamide gels. The molecular weight markers (lane Mr ) in (B) are, from top to bottom, 200, 97, 68, and 46 kD. Intact FBG (340 kD) is denoted by the arrow.
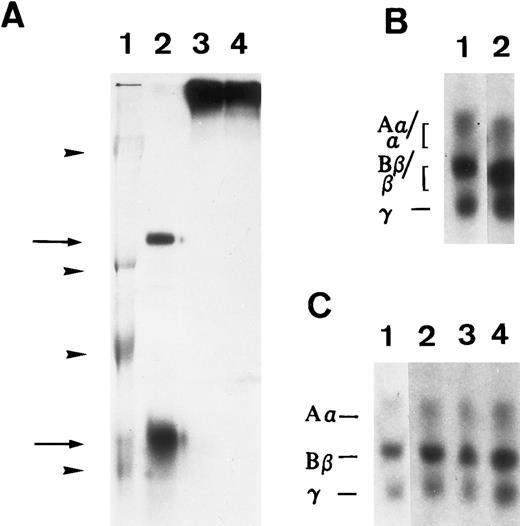
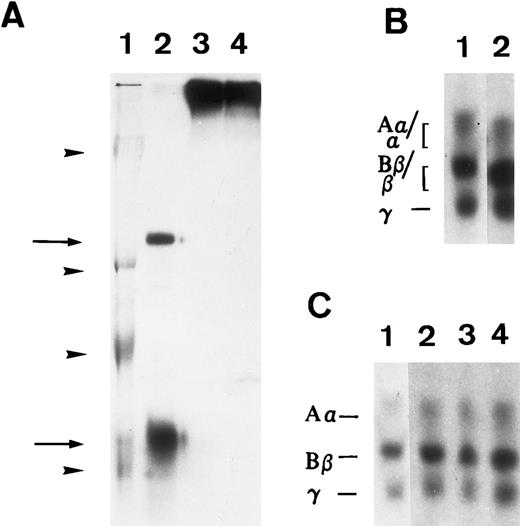
Susceptibility of iodinated plasma FBG to thrombin and plasmin action in A549 cell medium in the presence or absence of serum. 125I-labeled FBG was added to serum-free medium and treated with plasmin (A) or thrombin (B) or to A549 conditioned 10% serum-containing medium (C). (A) represents an autoradiograph of a nonreduced, 8% polyacrylamide gel. (B) and (C) represent 7% polyacrylamide gels of reduced fibrin(ogen) polypeptide chains. (A) Lane 1, Mr denoted by arrowheads are, from top to bottom, 200, 97, 68, and 46 kD; lane 2, plasmin-cleaved 125I-FBG fragments D and E (arrows, top to bottom); lane 3, 125I-FBG plasmin-treated in the presence of 200 U/mL aprotinin; lane 4, 125I-FBG starting material. (B) Lane 1, untreated 125I-FBG Aα, Bβ, and γ chains; lane 2, thrombin-cleaved fibrin α, β, and γ chains. (C) Lane 1, 125I-FBG in serum-free medium; lane 2, 125I-FBG + hirudin and aprotinin; lane 3, 125I-FBG + hirudin; lane 4, 125I-FBG + aprotinin.
Susceptibility of iodinated plasma FBG to thrombin and plasmin action in A549 cell medium in the presence or absence of serum. 125I-labeled FBG was added to serum-free medium and treated with plasmin (A) or thrombin (B) or to A549 conditioned 10% serum-containing medium (C). (A) represents an autoradiograph of a nonreduced, 8% polyacrylamide gel. (B) and (C) represent 7% polyacrylamide gels of reduced fibrin(ogen) polypeptide chains. (A) Lane 1, Mr denoted by arrowheads are, from top to bottom, 200, 97, 68, and 46 kD; lane 2, plasmin-cleaved 125I-FBG fragments D and E (arrows, top to bottom); lane 3, 125I-FBG plasmin-treated in the presence of 200 U/mL aprotinin; lane 4, 125I-FBG starting material. (B) Lane 1, untreated 125I-FBG Aα, Bβ, and γ chains; lane 2, thrombin-cleaved fibrin α, β, and γ chains. (C) Lane 1, 125I-FBG in serum-free medium; lane 2, 125I-FBG + hirudin and aprotinin; lane 3, 125I-FBG + hirudin; lane 4, 125I-FBG + aprotinin.
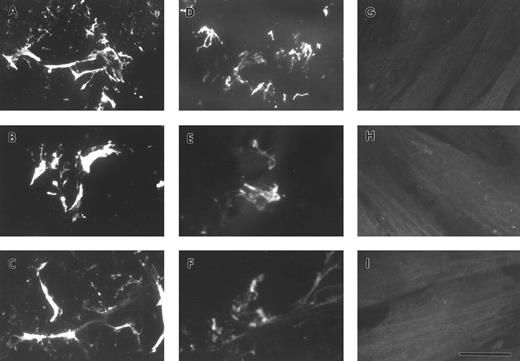
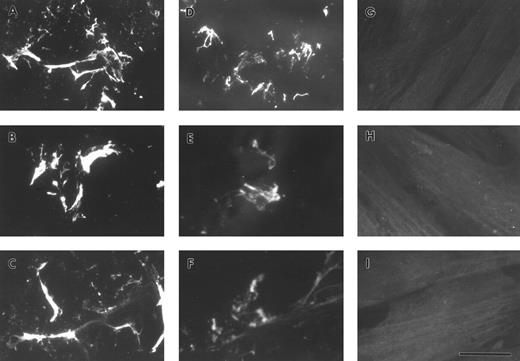
A549 cell-derived and purified plasma FBG binds to exogenously produced ECM.To determine whether lung epithelial cell-derived FBG could bind to exogenously produced ECM, binding of FBG to cultured HFF was studied. A549 cells were treated with IL-6 + DEX for 18 hours, and the conditioned medium was collected, overlaid onto confluent monolayers of fibroblasts, and then incubated for 72 hours at 37°C. Fibroblast cultures were also incubated for 72 hours with purified plasma FBG in HFF-conditioned media. Immunostaining of HFF using the fibrin(ogen)-specific MoAb, J88B, in the absence of permeabilization showed staining in a fibrillar pattern characteristic of ECM. Fibrin(ogen) staining in matrix was seen after incubation of HFF with both A549 cell-derived FBG (Fig 5A) and purified plasma FBG at 50 (Fig 5B), 10, and 2 μg/mL (not shown), but was absent from HFF not exposed to exogenous FBG (Fig 6G through I). The FBG staining pattern observed was exclusively extracellular in the nonpermeabilized cells, because parallel staining using antibody against the intracellular protein, α-tubulin, showed no positive staining (Fig 5C).
Exogenously produced FBG deposition in preformed matrix of fibroblasts. A549 cell conditioned media (A) or purified plasma FBG (B) was added to confluent monolayers of HFFs grown on glass coverslips and then cultured for 3 days. The cells were fixed and stained, nonpermeabilized (A through C), with MoAb J88B for FBG (A and B) and an MoAb for α-tubulin (C). Permeabilized HFFs were stained with the MoAb for α-tubulin (D). The bar in (D) represents 15 μm.
Exogenously produced FBG deposition in preformed matrix of fibroblasts. A549 cell conditioned media (A) or purified plasma FBG (B) was added to confluent monolayers of HFFs grown on glass coverslips and then cultured for 3 days. The cells were fixed and stained, nonpermeabilized (A through C), with MoAb J88B for FBG (A and B) and an MoAb for α-tubulin (C). Permeabilized HFFs were stained with the MoAb for α-tubulin (D). The bar in (D) represents 15 μm.
Exposure of FBG and fibrin-specific epitopes on A549 lung cell-derived FBG and plasma FBG deposited in the ECM of fibroblasts. Immunofluorescent staining was performed using MoAbs specific for FPA (A, D, and G), FPB (B, E, and H), and fibrin β15-21 (C, F, and I). HFFs were overlaid with conditioned media from A549 cells (A through C), with complete media containing purified plasma FBG (D through F ), or with complete media with no added ligand. The bar in (I) represents 10 μm.
Exposure of FBG and fibrin-specific epitopes on A549 lung cell-derived FBG and plasma FBG deposited in the ECM of fibroblasts. Immunofluorescent staining was performed using MoAbs specific for FPA (A, D, and G), FPB (B, E, and H), and fibrin β15-21 (C, F, and I). HFFs were overlaid with conditioned media from A549 cells (A through C), with complete media containing purified plasma FBG (D through F ), or with complete media with no added ligand. The bar in (I) represents 10 μm.
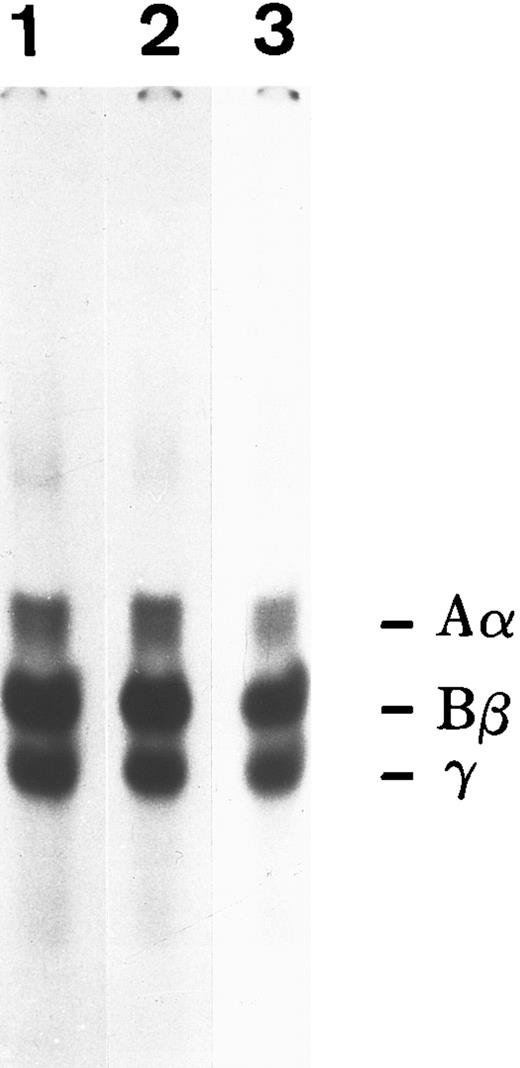
To ascertain if both FBG and fibrin-specific epitopes were exposed when bound to the ECM, immunostaining of HFF cultures incubated with A549 cell-derived and plasma FBG was performed with MoAbs RDV3, 18C6, and T2G1, which recognize native, fixed, denatured, and reduced FPA, FPB, and fibrin β15-21 (Table 1). Such staining indicated that both FBG- and fibrin-specific epitopes were indeed exposed (Fig 6). The presence of hirudin and aprotinin did not alter the staining pattern detected using any of these antibodies (not shown), suggesting that FBG deposition in the matrix of HFF does not involve the action of thrombin or plasmin. To further investigate the involvement of proteolytic processing during deposition of FBG into ECM, 125I-labeled purified plasma FBG (50 μg/mL) was overlaid onto HFF and incubated for 72 hours, and then monolayers were washed. Cell-associated (bound) and soluble (unbound) FBG was recovered by immunopurification and analyzed by SDS-PAGE (Fig 7). Electrophoretic mobility of reduced FBG Aα, Bβ, and γ chains (bound and unbound) was unaltered from that of native plasma FBG, further supporting the concept that incorporation into the matrix occurs in the absence of thrombin and plasmin cleavage, as well as in the absence of γ-γ dimer or α-polymer formation.
SDS-PAGE analysis of iodinated plasma FBG exposed to confluent monolayers of HFF for 3 days in culture. Lane 1, 125I-FBG starting material; lane 2, unbound FBG recovered from HFF media; lane 3, bound FBG recovered from cell monolayer.
SDS-PAGE analysis of iodinated plasma FBG exposed to confluent monolayers of HFF for 3 days in culture. Lane 1, 125I-FBG starting material; lane 2, unbound FBG recovered from HFF media; lane 3, bound FBG recovered from cell monolayer.
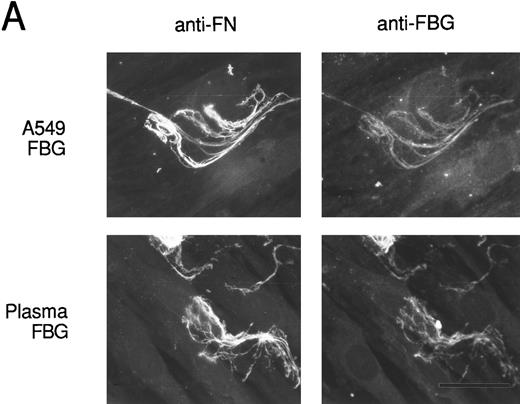
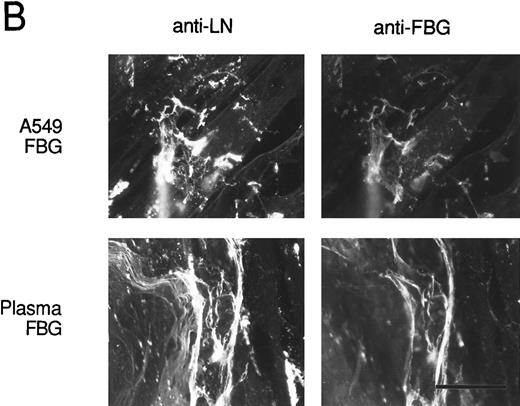
To verify that FBG colocalizes with other endogenously synthesized ECM proteins, double-label immunofluorescent staining was used. HFF incubated with A549 cell-conditioned medium or plasma FBG for 72 hours were stained first with PoAbs specific for FN or laminin (LN) and then with MoAb J88B to detect FBG. Both A549 cell-derived and plasma FBG clearly colocalized with fibrillar matrix strands containing either FN or LN (Fig 8).
Colocalization of FBG with ECM constituents in HFF matrix detected by dual immunofluorescent staining. FBG from lung A549 epithelial cells or plasma was overlaid onto HFF cultures for 3 days. Matrix fibrils were detected by dual staining with anti-FN and MoAb J88B (A) or anti-LN and MoAb J88B (B) antibodies. The bar represents 20 μm.
Colocalization of FBG with ECM constituents in HFF matrix detected by dual immunofluorescent staining. FBG from lung A549 epithelial cells or plasma was overlaid onto HFF cultures for 3 days. Matrix fibrils were detected by dual staining with anti-FN and MoAb J88B (A) or anti-LN and MoAb J88B (B) antibodies. The bar represents 20 μm.
To determine the protameric structure of these FBG fibril strands, the amount of DOC-soluble compared with DOC-insoluble FBG deposited into ECM was measured. A549-FBG from the DOC-soluble and -insoluble fractions was recovered by immunopurification and analyzed by SDS-PAGE. The fluorographs of the gels (not shown) were subjected to scanning densitometry to quantitate the relative amount of FBG in the DOC-insoluble compared with the -soluble fractions. After 2 hours of chase, the amount of DOC-insoluble FBG represented 8.6% of the A549 cell/matrix-associated fraction, which increased to 13.1% by 6 hours postlabeling and then declined to 5.4% by 24 hours. The amount of A549 cell-derived FN incorporated into DOC-insoluble material was 11.4% at 2 hours, 19.3% at 6 hours, and 8.7% at 24 hours, which closely paralleled the time course and amount of A549 cell-derived FBG incorporated into DOC-insoluble material.
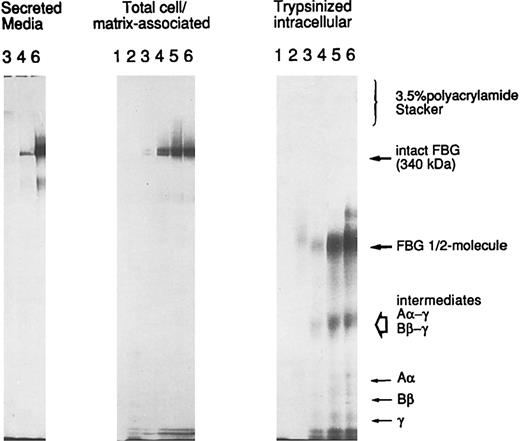
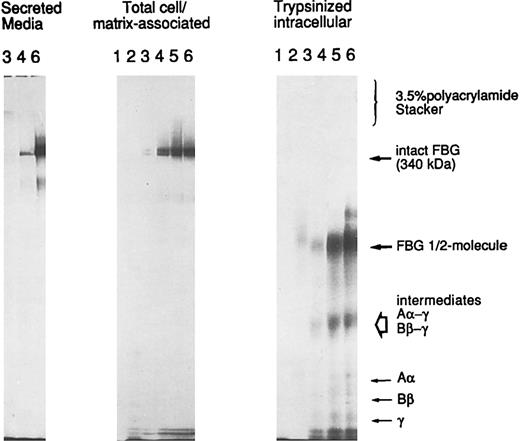
A549 cell-associated FBG is cell surface or matrix-associated.To determine the localization of A549 cell/matrix-associated FBG, cells induced with IL-6 + DEX were metabolically labeled over a short time course from 10 to 120 minutes. The washed cells were either lysed directly (cell/matrix-associated fraction) or first subjected to trypsinization to remove cell surface-bound proteins and then lysed in the standard cell lysing buffer used for immunopurification (intracellular fraction). The immunopurified FBG was analyzed by SDS-PAGE without thiol-reduction on a 6% polyacrylamide resolving gel with a 3.5% polyacrylamide stacking gel (Fig 9). Metabolically labeled FBG was not detectable in the cell/matrix-associated or trypsinized-intracellular fractions at the 10- or 20-minute time points. Intact FBG of 340 kD was first detectably cell/matrix-associated at 30 minutes, with undetectable levels of intact, labeled FBG in the corresponding intracellular pool derived from the trypsinized cells. In addition, labeled FBG was secreted into the culture media within 30 minutes (Fig 9), the time required for the secretion of newly synthesized and assembled FBG from HepG2 cells33 and A549 cells (unpublished data). As the labeling time increased, the amount of cell-associated FBG increased as well, with the majority remaining cell-surface bound and/or matrix-associated. The cell-bound FBG reached saturation between 60 and 90 minutes, with approximately 71% to 76% of the newly synthesized and secreted FBG remaining cell/matrix-associated. However, after reaching saturation of labeled FBG bound at the cell surface, the amount of FBG accumulating in the media increased. No high molecular weight aggregates were detected above FBG at 340 kD or in the stacking gel (Fig 9), consistent with the absence of high molecular weight aggregates of FBG in the DOC-soluble or -insoluble fractions analyzed by 2% agarose SDS-gel electrophoresis (not shown). In addition, no detectable intact FBG was immunopurified from the intracellular fraction of trypsinized cells, as would be expected for a rapidly secreted protein.33 Only free individual chains (Aα, Bβ, or γ), intermediate γ-β and γ-α chain complexes, and the FBG half-molecule (αβγ) were detected intracellularly (Fig 9; unpublished data). Thus, all of the cell-associated FBG is in the dimeric protamer form of 340 kD composed of the characteristic Aα, Bβ, and γ chains and not high molecular weight polymers. The FBG is extracellular, either bound to the surface of the cell and/or deposited into the ECM. Together, the data suggest that the mechanism of deposition of FBG closely parallels that of FN in A549 ECM.
Trypsin-sensitivity of cell/matrix-associated FBG. A549 cells were metabolically labeled with 35S-cys + 35S-met for 10 (lanes 1), 20 (lanes 2), 30 (lanes 3), 60 (lanes 4), 90 (lanes 5), and 120 (lanes 6) minutes. FBG secreted into the media was sampled at 30, 60, and 120 minutes (left panel); total FBG remaining cell-associated (middle panel) and intracellular FBG remaining after trypsinization of cell monolayers (right panel) were sampled at all time points, immunopurified, and resolved under nonreducing SDS-PAGE conditions. The position of migration of secreted and cell-bound intact FBG (disulfide-bonded Aα, Bβ, and γ chains) is denoted at 340 kD. The intracellular intermediates of FBG were determined by two-dimensional gel electrophoresis (not shown) and are labeled accordingly.
Trypsin-sensitivity of cell/matrix-associated FBG. A549 cells were metabolically labeled with 35S-cys + 35S-met for 10 (lanes 1), 20 (lanes 2), 30 (lanes 3), 60 (lanes 4), 90 (lanes 5), and 120 (lanes 6) minutes. FBG secreted into the media was sampled at 30, 60, and 120 minutes (left panel); total FBG remaining cell-associated (middle panel) and intracellular FBG remaining after trypsinization of cell monolayers (right panel) were sampled at all time points, immunopurified, and resolved under nonreducing SDS-PAGE conditions. The position of migration of secreted and cell-bound intact FBG (disulfide-bonded Aα, Bβ, and γ chains) is denoted at 340 kD. The intracellular intermediates of FBG were determined by two-dimensional gel electrophoresis (not shown) and are labeled accordingly.
DISCUSSION
FBG has been long regarded as serving a hemostatic role because it is converted from a soluble, plasma protein to an insoluble fibrin gel by the action of thrombin and factor XIIIa.2,4 Previous studies have identified fibrillar strands within the provisional matrix of cutaneous and vascular wounds as fibrin.34 During coagulation, additional adhesive glycoproteins from plasma become incorporated into the fibrin clot by covalent cross-linking, providing, in addition to the hemostatic plug, a scaffold for cell migration and proliferation, a reservoir for growth factors, proteases, and protease inhibitors, and a substrate for induction and modulation of cell function.34-37 Once re-epithelialization is complete or vascular integrity reestablished, fibrin is dissolved through the action of plasmin. Recently, we showed that lung epithelial cells synthesize and secrete FBG basolaterally in response to IL-6 + DEX.16,17 Furthermore, our earlier studies indicate that Pneumocystis carinii infection results in induction of FBG gene expression in lung epithelial cells in addition to its induction in hepatocytes.38 It is conceivable, therefore, that FBG synthesized at the site of wound repair or derived from plasma due to increased vascular permeability may incorporate into the provisional matrix independently of conversion to fibrin. The unexpected expression of FBG in extrahepatic cells led us to explore its role in cellular processes distinct from hemostasis. We show here deposition of lung epithelial cell FBG into extracellular matrices and its colocalization with other matrix proteins, FN and LN.
The mechanism of FBG assembly into matrix is presently not known; however, our experimental data suggest that FBG assembly in matrix may share several features with FN assembly in matrix. An accepted model of FN assembly begins with dimeric (protameric) FN binding to cell surface α5β1 via the RGD-containing cell adhesive region in FN repeats type III-8-10. Binding of FN to α5β1 results in a conformational change that enables a distinct cell surface component, the FN matrix assembly receptor, to bind the N-terminal (type I-1-5) modules of FN with high avidity. This binding facilitates disulfide cross-linking and fibril elongation, which is also facilitated by cell movement. Additional FN molecules add to the growing fibril via the homophilic binding site in the type I-9/III-1 modules (reviewed in Mosher et al39 ). Recent evidence implicates FN binding to α3β1 via an alternative mechanism that bypasses FN binding to α5β1 in matrix assembly.40 The results of this study show that the majority of the newly synthesized FBG remains cell/matrix-associated in a trypsin-sensitive fraction, a portion of which becomes incorporated over time into a detergent-insoluble matrix fraction such as the ECM constituent FN.31,32 41
A number of other similarities between FN and lung epithelial cell FBG incorporation onto matrix were found. Both lung epithelial cell-derived FBG17 and endothelial cell-derived FN are secreted basolaterally.42 This polarized secretion of FN is correlated with the localization of high-affinity FN binding sites on the basolateral but not apical face of the endothelial cell.42 Both cellular and plasma FN are known to incorporate into ECM of heterologous cell types.32,43 Similarly, lung cell-derived FBG, as well as purified plasma FBG, bind to the ECM of a heterologous cell type, namely fibroblasts. The deposition of FN into the ECM results in an extended conformer that evokes expression of latent cell binding domains in the RGDS-containing region in addition to RGDS. This conformational alteration enhances the avidity of cell binding to matrix FN over soluble FN.44 We show by a number of approaches that the deposition of FBG in the ECM occurs in the absence of plasmin degradation, thrombin cleavage, or covalent γ-γ or α-polymer glutamyl-lysyl cross-linking associated with insoluble fibrin(ogen) polymer formation. Taken together, the data indicate that it is likely FBG and not fibrin that is the ECM component identified.
That the fibrin-specific epitope β15-21 is exposed in matrix-associated FBG, without demonstrable thrombin cleavage or plasmin degradation, leads to the notion that the FBG in the matrix is conformationally altered. The concept of novel binding site exposure on FBG has been previously demonstrated. FBG adsorption to various surfaces45-48 or binding to the platelet membrane glycoprotein αIIbβ336,38,39 has been found to induce exposure of MoAb binding sites not recognized on native, soluble plasma FBG. The binding of several MoAbs is enhanced after plasmin digestion of FBG,46,48,49 suggesting that accessibility of the epitopes is due to conformational changes associated with plasmin degradation. Furthermore, the conformational changes induced by platelet αIIbβ3-receptor binding uncover otherwise latent epitopes containing the RGDF sequence of the FBG Aα chain49 and residues 373-385 of the γ chain.48 However, the epitope, β15-21, identified by T2G1 is not exposed on surface-bound FBG.29,50 Thus, the availability of this epitope suggests that FBG interacts with the ECM in a unique fashion. Several important biologic properties have been ascribed to exposure of residues β15-42 on the fibrin neo-N-terminus as a result of thrombin cleavage, including endothelial cell spreading,8 proliferation,51 and the induction of new capillary formation in vitro.52 In addition, thrombin cleavage exposes a cryptic heparin binding domain defined by residues β15-42,53 which mediates heparin-dependent binding of soluble fibrin fragments to endothelial cells.54 The T2G1 epitope is contained within this conformationally sensitive heparin binding domain of fibrin(ogen).53 Therefore, exposure of the T2G1 binding site during assembly of FBG in matrix may involve cell surface heparan sulfate proteoglycan-dependent binding interactions.
The results of this study support the concept that there is variability in the composition of provisional matrices deposited in response to inflammation and that different cell populations may preferentially require different matrix protein configurations to induce wound healing.34 Shifts in ECM proteins may also be influenced by the cell populations that migrate in and attach to the wound site. For example, FN synthesized by resident macrophages and fibroblasts during cutaneous wound repair is the embryonic form of the protein, suggesting that this particular structure of FN may promote or enhance wound healing in this context.55 The protein content of the matrix in liver, kidneys, heart, and lungs is altered in association with various pathologies.56 Enhanced deposition of plasma FN into the subendothelial matrix can decrease the protein permeability of lung endothelial monolayers in vitro as well as the lung endothelial barriers in vivo.57
In summary, the results presented in this study provide evidence that matrix-bound FBG may possess unique structural properties. We propose that inflammation induces the synthesis and deposition of FBG in ECM. This matrix-incorporated FBG assumes a new conformation, exposing a latent epitope that may result in new adhesive properties of surface-bound FBG to promote reciprocal cell-matrix processes.
ACKNOWLEDGMENT
The authors thank Brian J. Rybarczyk for preparation of fibronectin-free plasma fibrinogen, Sarah O. Lawrence for radioiodination of fibrinogen, and Dr Norma B. Lerner for critical comments and suggestions regarding the manuscript.
Supported by Public Health Service Grants No. HL50615, HL30616, and AI07362 from the National Institutes of Health (Bethesda, MD).
Address reprint requests to Patricia J. Simpson-Haidaris, PhD, Vascular Medicine, PO Box 610, 601 Elmwood Ave, Rochester, NY 14642.