Abstract
Previous studies showed that factor XI (FXI) deficiency commonly observed in Ashkenazi Jews is caused by two similarly frequent mutations, type II (Glu117stop) and type III (Phe283Leu) with allele frequencies of 0.0217 and 0.0254, respectively. In Iraqi Jews, who represent the ancient gene pool of Jews, only the type II mutation was observed with an allele frequency of 0.0167. In this study we sought founder effects for each mutation by examination of four FXI gene polymorphisms enabling haplotype analysis in affected Jewish patients of Ashkenazi, Iraqi, and other origins and in Arab patients. Initial population surveys of 387 Middle Eastern Jews (excluding Iraqi Jews), 560 North African/Sephardic Jews, and 382 Arabs revealed allele frequencies for the type II mutation of 0.0026, 0.0027, and 0.0065, respectively. In contrast, the type III mutation was not detected in any of these populations. All 60 independent chromosomes bearing the type III mutation were solely observed in Ashkenazi Jewish patients and were characterized by a relatively rare haplotype. All 103 independent chromosomes bearing the type II mutation in patients of Ashkenazi, Iraqi, Yemenite, Syrian, and Moroccan Jewish origin and of Arab origin were characterized by another distinct haplotype that was rare among normal Ashkenazi Jewish, Iraqi Jewish, and Arab chromosomes. These findings constitute the first example of a mutation common to Ashkenazi Jews, non-Ashkenazi Jews, and Arabs and are consistent with the origin of type II mutation in a founder before the divergence of the major segments of Jews. Our findings also indicate that the type III mutation arose more recently in an Ashkenazi Jewish individual.
COAGULATION FACTOR XI (FXI) deficiency is an autosomal recessive injury-related bleeding disorder first described as a “new” form of hemophilia.1 The disorder is highly prevalent among Jews mainly of Ashkenazi (European) origin and occurs only sporadically in other populations.2-8
The FXI (F11) gene is located on chromosome 4 (4q35).9 It is 23 kb long and is organized in 15 exons and 14 introns.10 Three mutations were initially identified in six Ashkenazi Jewish patients and were designated type I, type II, and type III.11 Type I mutation is a G to A substitution at the donor splice site in the last intron (intron N) of the FXI gene; type II is a nonsense mutation creating a premature stop codon (Glu117Stop) in exon 5, and type III is a Phe283Leu missense mutation in exon 9 coding for a protein that fails to be properly secreted from cells.12 Homozygotes for type III mutation have a significantly higher level of FXI clotting activity (6% to 14% of normal) than homozygotes for type II mutation (0.5% to 1.5% of normal), as well as significantly fewer episodes of injury-related bleeding.13 Recently, we described another mutation in an Ashkenazi Jewish patient and designated it type IV mutation. It involves a 14 bp deletion at the exon 14/intron N junction of the FXI gene.14 A survey of 125 unrelated Ashkenazi Jewish patients with major FXI deficiency showed that type II and type III mutations were the predominant mutations in this ethnic group, each accounting for 49.2% of the mutant alleles. The frequency of type I and type IV mutations was only 1.2% and 0.4%, respectively.5
In recent years we observed several families of Iraqi Jewish and Arab origin with members affected by FXI deficiency that was caused by the type II mutation.13,15 A survey of the type II and type III mutations in Ashkenazi and Iraqi Jews residing in Israel revealed that the type II mutation is as frequent in Iraqi Jews as it is in Ashkenazi Jews, with allele frequencies of 0.0167 and 0.0217, respectively. The type III mutation was not detected in any of the Iraqi Jews examined, whereas in Ashkenazi Jews its allele frequency was 0.0254.5 Iraqi Jews constitute the largest subgroup of contemporary Middle Eastern Jews. They are thought to represent the original Middle Eastern Jewish gene pool because they had lived in relative isolation from the times of the Babylonian exile in 586 BC until their immigration to Israel in 1950 to 1951. Ashkenazi Jews are also thought to have stemmed from the original pool, particularly after the destruction of the Second Temple in 70 AD. They have consolidated into a distinct ethnic entity in Europe in the ninth century AD.16 Sephardic Jews constitute the third large segment of the original Middle Eastern pool. They have migrated over the centuries along North Africa into Sepharad (Spain in Hebrew), and following their expulsion from Spain in 1492 have migrated back to North Africa, Italy, the Balkan countries, and Turkey.
Given the historical information and our finding of a similar prevalence of the type II FXI mutation in Ashkenazi and Iraqi Jews, we speculated that this mutation might have originated in an ancient Jewish founder.5 We also hypothesized that the type III mutation stemmed from a founder who lived at a time when the Ashkenazi Jews had already become a distinct ethnic community. In the present study we addressed these questions directly by analyzing FXI gene polymorphisms in deficient patients and control individuals of Iraqi and Ashkenazi Jewish as well as of Arab origin. We also surveyed other Middle Eastern Jews, Sephardic Jews, and Arabs for the type II and type III mutations.
MATERIALS AND METHODS
Subjects with FXI deficiency and their family members.Probands affected by FXI deficiency were previously diagnosed following referral for an injury-related bleeding episode or for a work-up of an occasional finding of prolonged activated partial thromboplastin time. Other FXI-deficient subjects or unaffected individuals were identified during examination of family members of probands. Diagnosis of FXI deficiency was based on finding of a diminished plasma factor XI activity, which in homozygotes or compound heterozygotes for the various mutations was less than 15 U/dL, and in heterozygotes from 22 to 60 U/dL.17 Genotype definition was established by analysis of type I through type IV mutations as described elsewhere.5 14
Eighty-three probands (from 80 families) were of Ashkenazi Jewish origin, 7 probands were of Iraqi Jewish origin (7 families), 6 probands were of Arab origin (from 4 Moslem and 2 Christian families), and one proband was of Yemenite Jewish origin.
Control subjects of Sephardic/North African and Middle Eastern Jewish and of Arab origins.Patients admitted to the Departments of Medicine or Surgery of the Sheba, Hasharon, Rambam, Assaf Harofeh, Rabin, and Sourasky Tel-Aviv Medical Centers or to Rosh-Haayin community clinic were examined for the common type II and type III mutations causing FXI deficiency in Jews. The study was approved by the human subject ethics committee. Definition of ethnic origin was based on the country of birth of the individual's four grandparents. The following groups of subjects were categorized: Sephardic/North African Jews, consisting of subjects of Moroccan, Algerian, Tunisian, Lybian, Turkish, and Greek origins; Middle Eastern Jews, comprising subjects of Iranian, Yemenite, and Syrian origin; and Arabs. Iraqi and Ashkenazi Jews were previously sampled and classified as separate groups.5
Analysis of four polymorphic markers in the FXI gene.DNA was prepared from fresh or frozen blood by the desalting procedure.18 A previously described restriction fragment length polymorphism (RFLP) in intron E and a dinucleotide repeat polymorphism in intron B were analyzed by polymerase chain reaction (PCR) as reported.19 20 Two new polymorphisms were discovered during the progress of this study. One was a single stranded conformation polymorphism (SSCP) in intron A. For its detection the following primers were designed: forward primer, derived from an intron A sequence (5′-TCAGATGGTGGCCATGAGAAGG-3′); and a reverse primer, derived from an intron B sequence (5′-TTCACCTCAATTCCTACCCTGC-3′). The 544-bp amplified fragment that was obtained consisted of 317 bp of intron A, 56 bp of exon 2, and 171 bp of intron B of the FXI gene. Although the exact change of sequence responsible for this poymorphism has yet to be determined, its location is most probably in intron A. This is supported by the detection of the SSCP in a 330-bp fragment derived from the 5′ end of the 544-bp amplified fragment by Fokl digestion. The 330-bp fragment contains 317 bp of intron A and 13 bp of exon 2 corresponding to the Fokl restriction site.
The discovery of the second new polymorphism emanated from the observation of 10 AT repeats in intron M of the reported FXI gene sequence.10 PCR using a forward primer, 5′-CTGAGATTGCAGCACTGCAC-3′, and a reverse primer, 5′-GTTCGTTTGTAGAATCATAC-3′, flanking this region disclosed four distinct alleles.
PCRs for the detection of the new intron A and intron M polymorphisms were performed in a total volume of 10 μL containing 50 ng of genomic DNA, 5 pmol of each primer, 20 μmol/L of each dNTP, 0.1 μL 32p-dATP (2,000 Ci/mmol), and 0.25 U Taq polymerase obtained from Advanced Biotechnologies (Surrey, UK). The amplification reaction for intron A was started by denaturation at 94°C for 5 minutes and followed by 30 cycles consisting of denaturation at 95°C for 30 seconds, annealing at 66°C for 45 seconds and extension at 72°C for 90 seconds. For amplification of the intron M polymorphic fragment, initial denaturation at 94°C was followed by 40 cycles consisting of denaturation at 98°C for 15 seconds, annealing at 50°C for 20 seconds, and extension at 72°C for 15 seconds. At the end of both PCRs, 5-minute extensions at 72°C were performed. The samples were then prepared for electrophoresis by adding 9 μL aliquots to 20 μL stop solution (95% formamide, 10 mmol/L NaOH, 0.25% bromphenol blue, 0.25% xylene cyanol), heating at 94°C for 5 minutes, and snap-chilling in ice water. In the SSCP assay of intron A, the PCR product was resolved by electrophoresis in 5% mutation detection enhancement (MDE) gel (AT Biochemical, Malvern, PA) at 6W constant power for 14 to 16 hours at room temperature. The amplification products of intron M were resolved by electrophoresis in a 6% polyacrylamide sequencing gel operated at 2,500 V for 3 hours. Gels were dried and visualized by autoradiography. The allele frequencies of the four polymorphic markers were determined in independent chromosomes of individuals belonging to the studied families whose FXI genotypes were normal and in samples of the Iraqi Jewish and Arab subjects surveyed for the type II mutation.
Derivation of FXI haplotypes.Haplotypes were derived as follows: (1) By analysis of the FXI gene polymorphisms in 17 unrelated homozygotes for the type II mutation and 13 unrelated homozygotes for the type III mutation; (2) by segregation analysis of polymorphisms in 292 members of 67 unrelated informative families in whom FXI-deficient patients were detected; (3) by segregation analysis of the polymorphisms in members of two Iraqi Jewish and four Arab families with patients affected by other inherited coagulopathies; and (4) by analysis of samples of Iraqi Jews and Arabs who were found to be homozygous for at least 3 of the 4 polymorphisms.
RESULTS
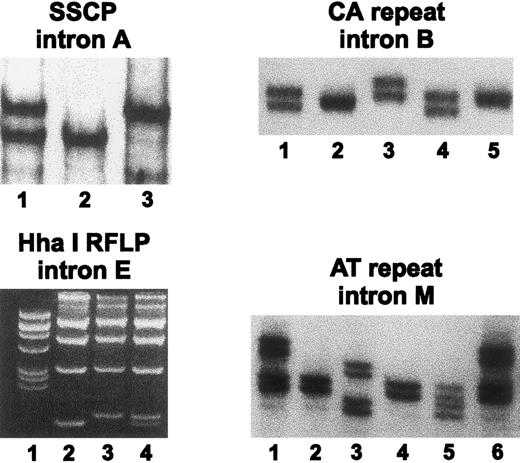
Characterization of the four FXI gene polymorphisms.Figure 1 depicts representative analyses of the four polymorphisms examined, and Table 1 summarizes the frequencies of the respective alleles in normal Ashkenazi Jewish, Iraqi Jewish, and Arab chromosomes. For the SSCP in intron A, two alleles were identified. The polymorphic information content (PIC) of this polymorphism was 0.34, 0.37, and 0.37 in Ashkenazi Jews, Iraqi Jews, and Arabs, respectively. For the intron B CA repeat polymorphism, we observed four alleles instead of two alleles originally reported.20 PIC values for this polymorphism were 0.58 in Ashkenazi Jews, 0.61 in Iraqi Jews, and 0.53 in Arabs. For the two allelic intron E RFLP, the PIC values were 0.36 in both Ashkenazi and Iraqi Jews and 0.37 in Arabs. For the intron M polymorphism, four alleles were detected. PIC values were 0.30, 0.34, and 0.17 in Ashkenazi Jews, Iraqi Jews, and Arabs, respectively.
Analysis of four polymorphic markers in introns A, B, E, and M of the FXI gene. SSCP in intron A: an autoradiogram shows samples of a heterozygote for alleles 1/2 (lane 1), a homozygote for allele 2 (lane 2), and a homozygote for allele 1 (lane 3). CA repeat in intron B: an autoradiogram shows samples of a heterozygote 2/4 (lane 1), a homozygote for allele 3 (lane 2), a heterozygote 1/2 (lane 3), a heterozygote 2/4 (lane 4), and a homozygote for allele 2 (lane 5). RFLP in intron E: an ethidium bromide–stained agarose gel shows in lane 1 the pBR322 DNA/Alul marker, samples of homozygotes for allele 2 (lane 2) and allele 1 (lane 3), and a sample of a heterozygote for alleles 1/2 (lane 4). AT repeat in intron M: an autoradiogram shows samples of a heterozygote for alleles 1 and 3 (lane 1), a homozygote for allele 3 (lane 2), a heterozygote for alleles 2 and 4 (lane 3), a homozygote for allele 3 (lane 4), a heterozygote for alleles 3 and 4 (lane 5), and a heterozygote for alleles 1 and 3 (lane 6).
Analysis of four polymorphic markers in introns A, B, E, and M of the FXI gene. SSCP in intron A: an autoradiogram shows samples of a heterozygote for alleles 1/2 (lane 1), a homozygote for allele 2 (lane 2), and a homozygote for allele 1 (lane 3). CA repeat in intron B: an autoradiogram shows samples of a heterozygote 2/4 (lane 1), a homozygote for allele 3 (lane 2), a heterozygote 1/2 (lane 3), a heterozygote 2/4 (lane 4), and a homozygote for allele 2 (lane 5). RFLP in intron E: an ethidium bromide–stained agarose gel shows in lane 1 the pBR322 DNA/Alul marker, samples of homozygotes for allele 2 (lane 2) and allele 1 (lane 3), and a sample of a heterozygote for alleles 1/2 (lane 4). AT repeat in intron M: an autoradiogram shows samples of a heterozygote for alleles 1 and 3 (lane 1), a homozygote for allele 3 (lane 2), a heterozygote for alleles 2 and 4 (lane 3), a homozygote for allele 3 (lane 4), a heterozygote for alleles 3 and 4 (lane 5), and a heterozygote for alleles 1 and 3 (lane 6).
Allele Frequencies of FXI Gene Polymorphisms in Ashkenazi and Iraqi Jews and in Arabs
| Polymorphism* . | Frequency† . | Arabs . | ||
|---|---|---|---|---|
| Intron . | Allele . | Ashkenazi Jews . | Iraqi Jews . | . |
| A | 1 | 0.32 | 0.47 | 0.41 |
| 2 | 0.68 | 0.53 | 0.59 | |
| (n = 128) | (n = 104) | (n = 91) | ||
| B | 1 | 0.02 | 0 | 0 |
| 2 | 0.37 | 0.42 | 0.38 | |
| 3 | 0.23 | 0.23 | 0.16 | |
| 4 | 0.38 | 0.35 | 0.47 | |
| (n = 134) | (n = 102) | (n = 116) | ||
| E | 1 | 0.39 | 0.39 | 0.45 |
| 2 | 0.61 | 0.61 | 0.55 | |
| (n = 137) | (n = 132) | (n = 126) | ||
| M | 1 | 0 | 0 | 0.01 |
| 2 | 0.16 | 0.13 | 0.18 | |
| 3 | 0.80 | 0.67 | 0.68 | |
| 4 | 0.04 | 0.20 | 0.13 | |
| (n = 131) | (n = 141) | (n = 103) | ||
| Polymorphism* . | Frequency† . | Arabs . | ||
|---|---|---|---|---|
| Intron . | Allele . | Ashkenazi Jews . | Iraqi Jews . | . |
| A | 1 | 0.32 | 0.47 | 0.41 |
| 2 | 0.68 | 0.53 | 0.59 | |
| (n = 128) | (n = 104) | (n = 91) | ||
| B | 1 | 0.02 | 0 | 0 |
| 2 | 0.37 | 0.42 | 0.38 | |
| 3 | 0.23 | 0.23 | 0.16 | |
| 4 | 0.38 | 0.35 | 0.47 | |
| (n = 134) | (n = 102) | (n = 116) | ||
| E | 1 | 0.39 | 0.39 | 0.45 |
| 2 | 0.61 | 0.61 | 0.55 | |
| (n = 137) | (n = 132) | (n = 126) | ||
| M | 1 | 0 | 0 | 0.01 |
| 2 | 0.16 | 0.13 | 0.18 | |
| 3 | 0.80 | 0.67 | 0.68 | |
| 4 | 0.04 | 0.20 | 0.13 | |
| (n = 131) | (n = 141) | (n = 103) | ||
See Fig 1.
N indicates the number of chromosomes examined.
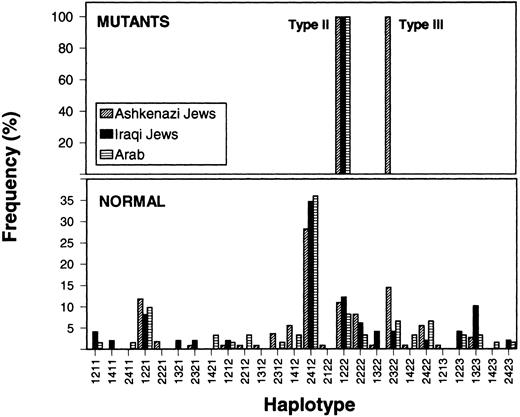
Founder effects for type II and type III mutations.The frequency distribution of haplotypes in control and mutant chromosomes is shown in Fig 2. The 26 identifiable haplotypes in control Iraqi Jewish, Ashkenazi Jewish, and Arab chromosomes were similarly distributed and one of them, ie, 2-4-1-2 predominated in all three populations.
Frequency distribution of FXI gene haplotypes observed in Ashkenazi Jews, Iraqi Jews, and Arabs. The numbers on the abcissa denote from bottom to top the allele numbers of polymorphisms in introns A, B, E, and M (as detailed in the legend of Fig 1). For instance, the haplotype designated 1211 is composed of allele 1 of intron A polymorphism, allele 2 of intron B polymorphism, allele 1 of intron E polymorphism, and allele 1 of intron M polymorphism. The lower and upper panels represent normal chromosomes and chromosomes bearing type II or type III mutations, respectively. Note that all chromosomes carrying the type II mutation are characterized by the same 1-2-2-2 haplotype that is observed in 8% to 12% of normal chromosomes. The chromosomes bearing the type III mutation are confined to Ashkenazi Jews, all characterized by haplotype 2-3-2-2.
Frequency distribution of FXI gene haplotypes observed in Ashkenazi Jews, Iraqi Jews, and Arabs. The numbers on the abcissa denote from bottom to top the allele numbers of polymorphisms in introns A, B, E, and M (as detailed in the legend of Fig 1). For instance, the haplotype designated 1211 is composed of allele 1 of intron A polymorphism, allele 2 of intron B polymorphism, allele 1 of intron E polymorphism, and allele 1 of intron M polymorphism. The lower and upper panels represent normal chromosomes and chromosomes bearing type II or type III mutations, respectively. Note that all chromosomes carrying the type II mutation are characterized by the same 1-2-2-2 haplotype that is observed in 8% to 12% of normal chromosomes. The chromosomes bearing the type III mutation are confined to Ashkenazi Jews, all characterized by haplotype 2-3-2-2.
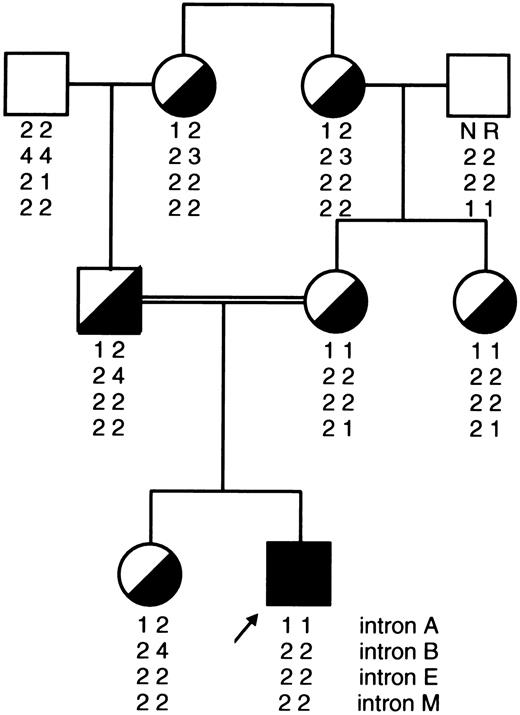
All independent chromosomes bearing the type II mutation, which were of Ashkenazi Jewish (n = 80), Iraqi Jewish (n = 10), and Arab (n = 11) origins, were characterized by a single haplotype, ie, 1-2-2-2. The same haplotype was associated with the type II mutation in a Yemenite Jewish family (Fig 3).
Pedigree of a Yemenite Jewish family depicting a proband with severe FXI deficiency (less than 1 U/dL) caused by homozygous type II mutation and six family members who are heterozygotes. It can be seen that the 1-2-2-2 haplotype segregates with the type II mutation as in Iraqi and Ashkenazi Jewish and Arab chromosomes bearing this mutation (Fig 2).
Pedigree of a Yemenite Jewish family depicting a proband with severe FXI deficiency (less than 1 U/dL) caused by homozygous type II mutation and six family members who are heterozygotes. It can be seen that the 1-2-2-2 haplotype segregates with the type II mutation as in Iraqi and Ashkenazi Jewish and Arab chromosomes bearing this mutation (Fig 2).
The haplotype analysis in type II heterozygotes detected during the population surveys yielded the findings that two heterozygotes of Syrian and Moroccan Jewish origins were homozygotes for the 1-2-2-2 haplotype. In 21 additional heterozygotes (14 of Iraqi, 1 of Yemenite, 1 of Moroccan, and 1 of Turkish Jewish origins and 4 of Arab origin) the polymorphic profile was also consistent with the association of the type II mutation with haplotype 1-2-2-2. The 1-2-2-2 haplotype was relatively rare in control Ashkenazi Jewish (10.9%), Iraqi Jewish (12.2%), and Arab (8.2%) chromosomes (Fig 2).
Sixty independent chromosomes bearing the type III mutation, all of Ashkenazi Jewish origin, were also characterized by a single yet another haplotype, ie, 2-3-2-2 (Fig 2). Interestingly, this haplotype was more frequent in normal Ashkenazi Jewish chromosomes (14.5%) than in normal Iraqi Jewish chromosomes (4.1%) and normal Arab chromosomes (6.1%).
Frequency of type II and type III mutations in Arabs and Sephardic/North African and Middle Eastern Jews.Among 841 Jewish subjects of Sephardic/North African or Middle Eastern origin and 313 Arab subjects, none was found to bear the type III mutation (Table 2). These data are similar to a previous survey of Iraqi Jews and contrast the relatively high frequency (0.0254) of the type III mutation in Ashkenazi Jews.5
Frequency of FXI Type II and Type III Mutations in Israeli Ethnic Groups
| Ethnic Group . | Type II Mutation . | Type III Mutation . | ||||||
|---|---|---|---|---|---|---|---|---|
| . | N . | Heterozygotes . | Homozygotes . | Allele . | N . | Heterozygotes . | Homozygotes . | Allele . |
| . | . | . | . | Frequency . | . | . | . | Frequency . |
| Ashkenazi Jews* | 531 | 21 | 1 | 0.0217 | 531 | 25 | 1 | 0.0254 |
| Iraqi Jews* | 509 | 17 | 0 | 0.0167 | 502 | 0 | 0 | — |
| Other Middle Eastern Jews | 387 | 2 | 0 | 0.0026 | 387 | 0 | 0 | — |
| Sephardic/North African Jews | 560 | 3 | 0 | 0.0027 | 454 | 0 | 0 | — |
| Arabs | 382 | 5 | 0 | 0.0065 | 313 | 0 | 0 | — |
| Ethnic Group . | Type II Mutation . | Type III Mutation . | ||||||
|---|---|---|---|---|---|---|---|---|
| . | N . | Heterozygotes . | Homozygotes . | Allele . | N . | Heterozygotes . | Homozygotes . | Allele . |
| . | . | . | . | Frequency . | . | . | . | Frequency . |
| Ashkenazi Jews* | 531 | 21 | 1 | 0.0217 | 531 | 25 | 1 | 0.0254 |
| Iraqi Jews* | 509 | 17 | 0 | 0.0167 | 502 | 0 | 0 | — |
| Other Middle Eastern Jews | 387 | 2 | 0 | 0.0026 | 387 | 0 | 0 | — |
| Sephardic/North African Jews | 560 | 3 | 0 | 0.0027 | 454 | 0 | 0 | — |
| Arabs | 382 | 5 | 0 | 0.0065 | 313 | 0 | 0 | — |
Previously reported.5
Five type II heterozygotes were identified among 947 Sephardic/North African and Middle Eastern Jews. These five individuals were of Moroccan (two), Turkish, Yemenite, and Syrian origins. Thus, the type II mutation was 6 to 8 times less frequent in Sephardic/North African and Middle Eastern Jews than in Iraqi or Ashkenazi Jews (Table 2). Among 382 Arab subjects, five heterozygotes were detected yielding a type II allele frequency of 0.0065.
DISCUSSION
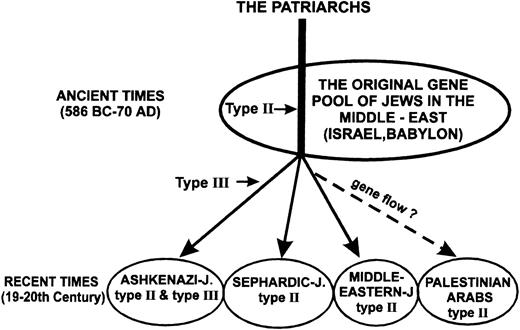
The presented data indicate that the type III mutation that causes FXI deficiency in Jews is confined to those of Ashkenazi origin. None of the 2,686 alleles examined of North African/Sephardic and Middle Eastern Jews was found to bear this mutation, nor was it observed in 626 alleles of Israeli Arabs (Table 2). We previously showed that the allele frequency of the type III mutation in Ashkenazi Jews is 0.0254.5 In this study we showed a founder effect for this mutation, with all 60 mutant alleles examined having the same 2-3-2-2 haplotype (Fig 2). This haplotype was observed in only 14.5% of normal Ashkenazi Jewish chromosomes. Taken together our findings strongly suggest that the type III mutation occurred in an Ashkenazi Jewish individual who lived at a time after the divergence of Ashkenazi Jews from their original Middle Eastern ancestors (Fig 4).
A simplified scheme showing the common origin of the three major segments of contemporary Jews and explaining the current distribution of the type II and type III mutations. The predicted time when type II and type III mutations occurred in the FXI gene are indicated by horizontal arrows. We speculate that gene flow has been responsible for the transfer of type II mutation from Middle Eastern Jews to Palestinian Arabs after the settlement of Arabs in Israel in the Seventh century AD.
A simplified scheme showing the common origin of the three major segments of contemporary Jews and explaining the current distribution of the type II and type III mutations. The predicted time when type II and type III mutations occurred in the FXI gene are indicated by horizontal arrows. We speculate that gene flow has been responsible for the transfer of type II mutation from Middle Eastern Jews to Palestinian Arabs after the settlement of Arabs in Israel in the Seventh century AD.
Unlike the type III mutation, the type II mutation is not confined to Ashkenazi Jews. In a previous study we showed that the allele frequency of the type II mutation was quite similar in Ashkenazi (0.0217) and Iraqi Jews (0.0167), the latter representing the original gene pool of Jews who have remained in the Middle East for 2,500 years.5 In this study we extended our observations to other Jewish ethnic groups and Arab individuals. Among 1,894 alleles of North African, Sephardic, and Middle Eastern Jewish origins (excluding Iraqi Jews), we detected only five that carried the type II mutation. This 6- to 8-times-lower allele frequency, when compared with the frequency in Iraqi and Ashkenazi Jews (Table 2), is reflected by our observation during the last 30 years of only one family of Yemenite Jewish origin with a severely affected patient who was homozygous for the type II mutation (Fig 3). Among Arabs we have encountered over the years, six unrelated families in whom patients with severe type II FXI deficiency manifested excessive bleeding. Conceivably, this is related to the somewhat higher prevalence of the mutant allele (0.0065) in the general Arab population when compared with the 0.0026 allele frequency in North African/Sephardic or non-Iraqi Middle Eastern Jews (Table 2).
Of great interest was the finding that all 103 informative independent chromosomes bearing the type II mutation of Ashkenazi, Iraqi, Yemenite, Syrian, and Moroccan Jews and of Arabs were characterized by one haplotype. This 1-2-2-2 haplotype (Fig 2) was observed in only 8% to 12% of normal chromosomes in Ashkenazi Jews, Iraqi Jews, and Arabs and hence the data are consistent with a founder effect.
The various Jewish individuals affected by the type II mutation belong to communities that diverged mainly during the 600 years that elapsed between the destruction of the First Temple in 586 BC and the destruction of the Second Temple in 70 AD (Fig 4). Consequently, it is likely that the type II mutation arose in an individual who lived in those ancient times or before. Gene flow might have been responsible for the transfer of the type II mutation to the Arabs who spread in the Middle East and occupied the land of Israel as of the Seventh century AD and later.
During the last decades a significant number of rare autosomal recessive disorders have been described among Jews of various origins.21 Most disorders are confined to one of the major segments of the Jews, namely Ashkenazi, Sephardic, or Middle Eastern, with none of the disorders being common to Ashkenazi and non-Ashkenazi Jews. Type II FXI deficiency is therefore the first disorder that is common to Ashkenazi and non-Ashkenazi Jews. The mechanisms involved in the emergence of the relatively high prevalence of the hereditary disorders in Ashkenazi and non-Ashkenazi Jews is still debated.22 Protagonists of the hypothesis of a selective advantage of heterozygotes base their arguments mainly on the phenomenal prevalence of four rare lysosomal disorders in Ashkenazi Jews and on the identification of multiple mutations causing them. However, in none of these disorders nor in patients with FXI deficiency of either type has an advantage been shown. Protagonists of the founder effect and drift hypothesis base their arguments on similar clustering of genetic disorders in the Amish, Finns, and French-Canadians; on the contractions and excessive expansions particularly of Ashkenazi Jews from the year 1500 and onwards; and on the absence of the specific mutations in populations that lived in proximity with Ashkenazi Jews.22,23 Recently, by determining the degree of linkage disequilibrium and the recombination frequency between the locus of idiopathic torsion dystonia and adjacent microsatellite markers, the time when the mutation causing this disease in Ashkenazi Jews took place was estimated to be around the year 1650.23 Work on dating the origin of other Ashkenazi Jewish disorders is in progress. At present there are only few characterized polymorphisms neighboring the FXI gene. When such markers become available it will be possible to estimate more precisely the time when the type II and type III mutations arose.
ACKNOWLEDGMENT
The authors are indebted to Professor Avinoam Adam of the Tel-Aviv University for useful discussion, to Rusa Eichel, Ety Zwang, and Rivki Yatuv for skillful technical assistance, and to Alice Nahum and Ruth Apel for excellent secretarial work.
Supported in part by grant No. 2601 of the Chief Scientist of the Ministry of Health of Israel.
Address reprint requests to Uri Seligsohn, MD, Institute of Thrombosis and Hemostasis, Department of Hematology, Sheba Medical Center, Tel-Hashomer, 52621 Israel.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal