Abstract
Clinical experience and laboratory data suggest that human cytomegalovirus (HCMV) is present in peripheral blood of seropositive individuals in a latent or persistent state and can be transmitted via blood products and be reactivated in seropositive imunocompromised patients. The pathophysiology of HCMV latency and the nature of HCMV interaction with hematopoietic cells remains unknown. In this study, we investigated the infection of bone marrow (BM) progenitor cells and their progeny as a model of HCMV latency. A clinical isolate and the recombinant laboratory strain Towne/lox containing the Escherichia coli β galactosidase (β-gal) gene regulated by immediately early (IE) HCMV promoter were used to infect highly purified CD34+ cells. Although the infection of these cells with a clinical isolate was associated with an inhibition of proliferation by 59%, an expansion of progeny derived from these cells was possible. Polymerase chain reaction analysis and staining for β-gal have shown that HCMV persisted in infected BM CD34+ cells and their progeny for up to 4 weeks. However, IE and late gene products (mRNA and protein) were detected only late in the course of infection and their expression correlated with terminal macrophage differentiation of the CD34+-derived progeny. Although early infection of CD34+ progenitor cells was not productive (as shown by the plaque assay), infectious virus could be recovered from the terminally differentiated cultures. BM progenitor cells may serve as a reservoir of the latent virus with limited transcription. Proliferation and monocytic maturation of infected progenitors may lead to the numerical expansion of HCMV-infected cells, which serve as a source of HCMV dissemination and reactivation.
PREVENTION OF HUMAN cytomegalovirus (HCMV) transmission in blood products and its reactivation in seropositive immunosuppressed patients remains a current problem with clinical and economic implications. Clinical experience and laboratory data suggest that HCMV is present in peripheral blood of seropositive individuals in a latent or persistent state.1-3 HCMV can be transmitted via blood products or organ transplantation and reactivated in immunocompromised patients.4-13
The identity of cells serving as a reservoir for HCMV has not been well characterized. Depletion of leukocytes from seropositive blood decreases the incidence of infection following transfusion,14-17 suggesting that the virus is present in one or more types of blood leukocytes. Monocytes are believed to be the main carriers of HCMV genome in seropositive healthy subjects.2,3 During an acute infection with HCMV, monocytes have been found to express HCMV antigens,18,19 and virus can readily be cultured from cells derived from viremic patients.20 However, there is little or no lytic gene expression in peripheral blood monocytes (PBM) of normal seropositive individuals and it is not possible to isolate HCMV from normal seropositive blood.21-23 In vitro infection of PBM by HCMV has shown viral expression only in a small number of cells and is restricted to immediate early events of the viral cycle.24,25 However, PBM become fully permissive for productive infection upon differentiation to macrophages.26-29 This evidence led to the hypothesis that monocytes may acquire HCMV earlier in their development in the bone marrow (BM). In agreement with this theory, suppression of the hematopoiesis is readily observed in the patients with disseminated HCMV infection.30-34 Clinically, HCMV infection has been associated specifically with failure of BM grafts, possibly through a direct viral effect on hematopoietic progenitor cells.31,35-37 Furthermore, in vitro HCMV infection has been shown to inhibit the proliferation and colony-forming ability of hematopoietic precursors.38 39
We hypothesized that latent HCMV is present in BM progenitor cells, with limited gene expression, and that these cells may be a source of viral transmission and activation. In our studies, we analyzed the HCMV life cycle in infected BM progenitor cells and their progeny to determine the mode of spread of latent HCMV under various clinical conditions.
MATERIALS AND METHODS
HCMV.CMV strains were propagated in human embryonic lung fibroblasts (HEL) maintained in Improved MEM Zink Option (Richter's Modification; GIBCO, Gaithersburg, MD) supplemented with 10% fetal bovine serum (GIBCO). Virus strains were passed at low virus to cell ratios to achieve a maximum titer of approximately 108 plaque-forming units (PFU)/mL. The 95(2) strain was isolated from patients with disseminated HCMV infection and has been maintained at a low passage level (obtained from Dr W.L. Drew, University of California, San Fransisco, CA). Recombinant strain Towne/lox2 was derived from the laboratory strain Towne and contains the LacZ gene of Escherichia coli regulated by the major IE HCMV promoter. Virus titers were determined by plaque assays using HEL cells as previously described.40
Detection of LacZ expression.X-gal (Boehringer Mannheim, Indianapolis, IN) was used as a substrate at a concentration of 1 mg/mL for detection of β-gal activity in viable cells at 1, 2, 3, and 4 weeks postinfection and in methylcellulose cultures of BM cells after a 14-day culture period. Controls included mock-infected cells and cells infected with virus lacking the LacZ gene.
Separation and culture of CD34+ cells.Normal BM cells were obtained by aspiration from the posterior iliac crest of healthy seronegative volunteers. Informed consent was obtained according to protocols approved by the Institutional Review Board of the University of Nevada, Reno Medical School. Mononuclear BM cells were isolated by density gradient centrifugation using lymphocyte separation medium (Organon, Durham, NC) and washed with Hank's Balanced Salt Solution (GIBCO). CD34+ cells were separated by avidin-biotin immunoadsorption using a CEPRATE LC CD34 cell selection KIT (Cell Pro, Inc, Bothel, WA) according to the manufacturer's protocols. Immediately after isolation, purified CD34+ cells were infected for 24 hours with the appropriate strains of HCMV at a high multiplicity of infection (100 PFU/cell). Mock-infected cells were incubated with the supernatant of virus stocks after infectious virus was pelleted by ultracentrifugation (105g for 60 minutes). After virus adsorption, the cells were washed three times to remove unattached virus and either cultivated in suspension in Iscove's Modification of Dulbecco's medium (GIBCO) supplemented with 20% fetal bovine serum, 50 ng/mL interleukin-3 (IL-3; Genzyme, Boston, MA), 50 ng/mL granulocyte colony-stimulating factor (G-CSF; Amgen, Thousand Oaks, CA), 50 ng/mL granulocyte-macrophage colony-stimulating factor (GM-CSF; Immunex Co, Seattle, WA), 50 ng/mL macrophage colony-stimulating factor (M-CSF ), 50 ng/mL stem cell factor (SCF; Amgen), 50 μg/mL L-glutamine and gentamycin (GIBCO BRL, Life Technologies, Inc, Grand Island, NY), or in methylcellulose supplemented with 50 ng/mL IL-3, 5 U/mL erythropoietin (EPO; Amgen), 50 ng/mL SCF, 50 ng/mL M-CSF, 50 ng/mL GM-CSF, 50 ng/mL G-CSF, or 10 U/mL EPO alone. Myeloid colonies were grown in methylcellulose supplemented with 100 ng/mL IL-3 and 100 ng/mL GM-CSF. The cells were plated at the density of 105 cells/mL in liquid cultures or at 7 × 103 cells per one well of a 6-well culture plate (Becton Dickinson Labware, Franklin Lakes, NJ) in methylcellulose. After 14 days, the colonies were counted under the light microscope. Media was changed every 4 to 5 days in liquid cultures and the cell counts were taken on a weekly basis. Purified BM CD34+ cells were incubated with anti-CD34 monoclonal antibodies (MoAbs) and developed with secondary goat antimouse IgG/IgM F(ab′) conjugated with Rhodamine-phycoerythrin (Becton Dickinson; Mountain View, CA) and then sorted by flow cytometry. Flow cytometric analysis of BM progenitor cells isolated from seronegative donors showed that the purity of sorted cells was 85% to 95% (data not shown).
DNA and RNA extraction.Colonies and cells from suspension cultures for nucleic acid analysis were washed in phosphate-buffered saline and total cellular DNA was extracted, after cell lysis with lysis buffer containing 0.2 mol/L Na-Acetate, 1% sodium dodecyl sulfate, and phenol/chloroform (24:1) followed with ethanol precipitation. Total cellular RNA was isolated by a standard guanidine isothyanate method.41
Polymerase chain reaction (PCR).HCMV DNA and RNA was detected using a nested reverse transcriptase-PCR (RT-PCR) method. Briefly, after isolation, RNA was treated with 5 U of Rnase-free Dnase (GIBCO BRL/Life Technologies, Inc) in the presence of 20 U of Rnase inhibitor (GIBCO BRL/Life Technologies, Inc) for 15 minutes at 37°C, and cDNA was synthesized using 0.5 μg Oligo dT primers (GIBCO BRL/Life Technologies, Inc), 1× PCR buffer (Promega, Madison, WI), and 200 U of M-MLV RT (GIBCO BRL/Life Technologies, Inc) in a 20 μL volume of reaction mix. The reaction proceeded for 1 hour at 42°C and was terminated by heating at 95°C for 6 minutes. Each PCR reaction mixture (30 μL) consisted of PCR buffer (50 mmol/L KCl, 10 μmol/L Tris-HCl [pH 8.3], 1.5 or 1.8 mmol/L MgCL2 , and 0.01% [wt/vol] gelatin), 200 μmol/L each deoxynucleoside triphosphate, 1 μmol/L each of the two amplification primers, and 2.5 U of Ampli Taq DNA polymerase (Promega). Each cycle consisted of denaturation at 94°C for 1 minute, annealing at 55°C or 52°C for 1 minute, and extension at 72°C for 2 minutes. Amplification cycles (35 or 40) were performed in a Perkin Elmer Cetus DNA Thermal Cycler followed by a final extension at 72°C for 7 minutes. The nested PCR reaction mixture (30 μL) consisted of 1 μL undiluted PCR product; all other components and the cycling parameters were the same as described above. Forty nested amplification cycles were performed. As a control for the presence of DNA or cDNA in each sample, β-actin primers were used. PCR products from the IE1 gene differed in length between DNA and cDNA samples. No introns are reported for the Major Capsid Protein (MCP) gene; therefore, the expected lengths of DNA and cDNA PCR products are the same. All primers used are depicted in Table 1. As controls, DNA and cDNA prepared from uninfected and infected HEL cells were used. Control samples for detection of accidental contamination contained all reagents except for DNA or cDNA. Several other controls have been used, including Dnase treatment of prepared RNA samples and preparations without cDNA synthesis (absent RT). The final amplification products were run on a 1% agarose gel containing ethidium bromide and were visualized with UV light.
Immunochemistry for detection of HCMV IE and late (L) antigens.Infected CD34+ progenitor cells were fixed in acetone/methanol (9:1) and reacted at a dilution of 1:100 with MoAb directed against IE1 or pp65 HCMV proteins (Chemicon International, Inc, Temicula, CA) for 1 hour at 37°C. The secondary antibody, goat antimouse alkaline phosphatase (AP) conjugate (Vector Laboratories, Inc, Burlingame, CA; 1:50) was reacted with the HCMV MoAb for 1 hour at 37°C, followed by incubation with a Vector Red (Vector AP substrate KIT; Vector Laboratories, Inc; containing an inhibitor of the intrinsic AP activity, levamisole) substrate for 15 minutes at 37°C.
For immunochemistry, a number of positive and negative controls were used. Positive controls consisted of infected permissive human foreskin fibroblasts (HFF) stained with specific antibodies. Negative controls consisted of infected and mock-infected cells incubated with secondary antibodies. In addition, in all experiments, a very stringent control was provided by the incubation of mock-infected cells with specific antibodies. Isotype controls were used only in initial experiment also yielding no background.
Cells were counter stained with methyl green (Vector) for 20 minutes, dehydrated, and mounted on the glass slides. The infected CD34+ cells or CD34+-derived monocytes and macrophages were grown on glass coverslips.
Detection of infectious virus.Plaque assays were conducted on HEL cells.40 Supernatants from infected CD34+ cells or CD34+-derived monocytes or macrophages were introduced into HEL cell cultures to recover infectious virus.
RESULTS
HCMV infection of BM progenitor cells.Previous studies showed that hematopoietic progenitor cells can be infected with some strains of HCMV and infected CD34+ cells express HCMV IE and L antigens in the early hours of infection.42 It is unclear whether HCMV persists upon proliferation and differentiation of these cells and whether HCMV gene products can be detected later during infection. To determine the expression pattern and kinetics of HCMV gene expression during infection of CD34+ BM cells and their progeny, long-term suspension cultures were performed. HCMV DNA, mRNA, and protein were assayed in cultures initiated with HCMV-infected highly purified CD34+ cells at 3, 7, 14, 21, and 28 days postinfection.
HCMV infected and mock-infected cells, harvested from liquid cultures, were screened by nested PCR for the presence of HCMV DNA and RNA at 3, 7, 14, 21, and 28 days of infection. HCMV DNA and unspliced mRNA were detected in CD34+ cultures infected with either a clinical isolate or recombinant laboratory strain Towne/lox2 at 3 days postinfection (Fig 1A).
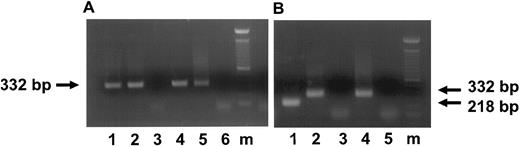
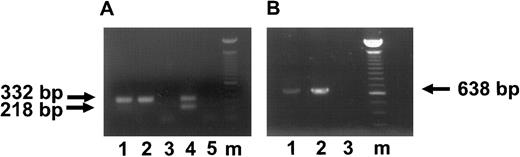
Detection of HCMV DNA and IE transcripts in HCMV-infected CD34+ progenitor cells 3 and 7 days postinfection. (A) Ethidium bromide-stained 1% agarose gel of PCR products for HCMV DNA (lanes 1 through 3) and of RT-PCR for IE transcripts (lanes 4 through 6; 3 days postinfection). The arrow indicates the IE PCR and RT-PCR products of 332 bp. Lane 1, DNA from CD34+ cells infected with HCMV strain 95(2); lane 2, DNA from CD34+ cells infected with Towne/lox2; lane 3, DNA from mock-infected CD34+ cells; lane 4, RNA from CD34+ cells infected with 95(2); lane 5, RNA frm CD34+ cells infected with Towne/lox2; lane 6, RNA from mock-infected CD34+ cells. (B) Ethidium bromide-stained 1% agarose gel of RT-PCR products of an IE transcripts (7 days postinfection) using primers spanning exons 1 and 2. The arrows indicate the RT-PCR products of spliced IE transcripts of 218 bp and unspliced IE transcripts of 332 bp. Lane 1, RNA from CD34+ cells infected with 95(2); lane 2, RNA from CD34+ cells infected with Towne/lox2; lane 3, RNA from mock-infected CD34+ cells; lane 4, positive control of HCMV-infected fibroblasts amplified from DNA displaying genomic size (332 bp) banding pattern; lane 5, RT-PCR H2O control. All RNA samples were treated with Rnase-free Dnase. Lane m, 100-bp ladder (GIBCO/BRL). Primers and predicted PCR products are listed in Table 1.
Detection of HCMV DNA and IE transcripts in HCMV-infected CD34+ progenitor cells 3 and 7 days postinfection. (A) Ethidium bromide-stained 1% agarose gel of PCR products for HCMV DNA (lanes 1 through 3) and of RT-PCR for IE transcripts (lanes 4 through 6; 3 days postinfection). The arrow indicates the IE PCR and RT-PCR products of 332 bp. Lane 1, DNA from CD34+ cells infected with HCMV strain 95(2); lane 2, DNA from CD34+ cells infected with Towne/lox2; lane 3, DNA from mock-infected CD34+ cells; lane 4, RNA from CD34+ cells infected with 95(2); lane 5, RNA frm CD34+ cells infected with Towne/lox2; lane 6, RNA from mock-infected CD34+ cells. (B) Ethidium bromide-stained 1% agarose gel of RT-PCR products of an IE transcripts (7 days postinfection) using primers spanning exons 1 and 2. The arrows indicate the RT-PCR products of spliced IE transcripts of 218 bp and unspliced IE transcripts of 332 bp. Lane 1, RNA from CD34+ cells infected with 95(2); lane 2, RNA from CD34+ cells infected with Towne/lox2; lane 3, RNA from mock-infected CD34+ cells; lane 4, positive control of HCMV-infected fibroblasts amplified from DNA displaying genomic size (332 bp) banding pattern; lane 5, RT-PCR H2O control. All RNA samples were treated with Rnase-free Dnase. Lane m, 100-bp ladder (GIBCO/BRL). Primers and predicted PCR products are listed in Table 1.
The recombinant virus Towne/lox2 was used to identify HCMV in liquid cultures formed from virus-infected hematopoietic progenitor cells. CD34+ BM cells in suspension cultures infected with the recombinant strain were analyzed for β-gal expression by X-gal staining. We were able to detect LacZ activity in 9% of CD34+ progenitors infected with the recombinant strain (data not shown). Consistent with this result, nested RT-PCR analysis using intron spanning primers (between exons 1 and 2) was capable of detecting unspliced transcripts from the HCMV major IE region in the cultures of CD34+ progenitor cells infected with both HCMV strains as early as 3 days postinfection (Fig 1A). This is consistent with a recent study that reported that the majority of IE region RNA appeared unspliced in HCMV infected granulocyte-macrophage progenitors during latency.43 However, analysis of the RNA from CD34+ progenitors infected with a clinical isolate showed spliced transcripts from HCMV IE region 7 days after infection, whereas IE RNA from CD34+ cells infected with recombinant laboratory strain collected at the same time after infection remained unspliced (Fig 1B). Although RT-PCR data confirmed the presence of major IE transcripts in infected BM progenitor cells, no evidence of viral protein expression (IE or L) was found in cultures of BM progenitor cells using immunocytochemistry.
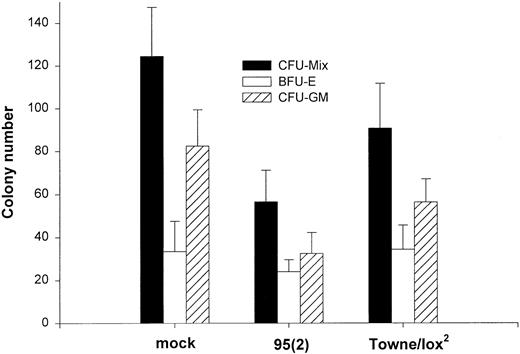
Effects of HCMV on the proliferation and differentiation of HCMV-infected BM progenitor cells.Previous experiments established the ability of selected HCMV strains to infect and persist in the cultures of immature hematopoietic cells characterized phenotypically by the expression of CD34+ antigen. Theoretically, HCMV infection of these cells may be lytic, leading to the destruction of affected cells, or may persist in a latent form causing more or less functional impairment. We therefore investigated whether infected progenitor cells retain the ability to proliferate and differentiate using methylcellulose and suspension cultures. BM cells were infected with either the clinical isolate [95(2)] or recombinant laboratory strain (Towne/lox2 ) for 24 hours or mock-infected. CD34+ progenitors were dispersed in methylcellulose immediately after virus adsorption at a density of 7 × 103 cells per well in 6-well culture plates. After 14 days in culture, total numbers of colonies were counted. Both virus strains produced inhibition of colony formation ability, ranging from 22% to 52% (Fig 2). However, the laboratory strain showed only a moderate inhibitory effect on the growth of BM progenitor cells (22%), whereas the recent clinical isolate inhibited colony formation by up to 52%. In addition to inhibition of erythroid (burst-forming unit-erythroid) and myeloid (colony-forming unit–granulocyte-macrophage) colonies, we observed a more striking suppression of the colony formation by the cells of the myeloid-monocytic lineage. This effect was observed with both strains of the virus used (Table 2).
Effect of HCMV on the colony formation by CD34+ BM cells. Purified CD34+ cells were either mock-infected or infected with 95(2) or Towne/lox2 at a multiplicity of infection (MOI) of 100 PFU/cell. A total of 7 × 103 cells were plated per well of 6-well plate in methylcellulose. The cultures were supplemented with 50 ng/mL SCF, 50 ng/mL IL-3, 50 ng/mL GM-CSF, 5 U/mL Epo, and 50 ng/mL M-CSF. Colonies were counted after 14 days in culture. Results represent the mean colony number ± SEM of the total amount of colonies (CFU-Mix), erythroid colonies (BFU-E), and myeloid colonies (CFU-GM) of four experiments performed.
Effect of HCMV on the colony formation by CD34+ BM cells. Purified CD34+ cells were either mock-infected or infected with 95(2) or Towne/lox2 at a multiplicity of infection (MOI) of 100 PFU/cell. A total of 7 × 103 cells were plated per well of 6-well plate in methylcellulose. The cultures were supplemented with 50 ng/mL SCF, 50 ng/mL IL-3, 50 ng/mL GM-CSF, 5 U/mL Epo, and 50 ng/mL M-CSF. Colonies were counted after 14 days in culture. Results represent the mean colony number ± SEM of the total amount of colonies (CFU-Mix), erythroid colonies (BFU-E), and myeloid colonies (CFU-GM) of four experiments performed.
Several studies showed that, in seropositive individuals, HCMV is predominantly present in monocytes.2,3,44 In suspension cultures designed to promote monocytic differentiation and proliferation, we investigated whether HCMV affects generation of mature monocytes from their precursors. Highly purified CD34+ BM cells derived from normal seronegative donors were inoculated with different strains of HCMV, and cultures were monitored for the number of viable cells using a trypan-blue exclusion test. As determined by the viability data (data not shown), no initial cytotoxic effect of HCMV on the CD34+ progenitors was detected in liquid cultures. When CD34+ BM progenitor cells were infected with a clinical isolate at high virus to cell ratios, significant inhibition of cell proliferation by HCMV was seen when compared with mock-infected control (Fig 3). Inhibition of BM cell proliferation ranged from 50% to 59%, depending on the time postinfection. Suppression peaked at 3 weeks postinfection. The observed inhibitory effect was not due to virus-mediated cytocidal effect, because no correlation was found between lack of cellular proliferation and cell viability (data not shown). In contrast, the laboratory strain tested resulted in no significant reduction in the cell proliferation when compared with mock-infected cultures. Furthermore, a significant induction of CD34+ cell proliferation infected with recombinant strain Towne/lox2 was observed in all four experiments performed.
Effect of HCMV on the generation of mature monocytes from their precursors in suspension cultures. Highly purified CD34+ cells were infected with 95(2) or with Towne/lox2 at MOI of 100 PFU/cell, or were mock-infected and grown in suspension cultures for 4 weeks. The cultures were supplemented with 50 ng/mL SCF, 50 ng/mL IL-3, 50 ng/mL GM-CSF, and 50 ng/mL M-CSF and monitored for a number of viable cells for 4 weeks using trypan-blue exclusion test. Each point represents the mean of four experiments performed.
Effect of HCMV on the generation of mature monocytes from their precursors in suspension cultures. Highly purified CD34+ cells were infected with 95(2) or with Towne/lox2 at MOI of 100 PFU/cell, or were mock-infected and grown in suspension cultures for 4 weeks. The cultures were supplemented with 50 ng/mL SCF, 50 ng/mL IL-3, 50 ng/mL GM-CSF, and 50 ng/mL M-CSF and monitored for a number of viable cells for 4 weeks using trypan-blue exclusion test. Each point represents the mean of four experiments performed.
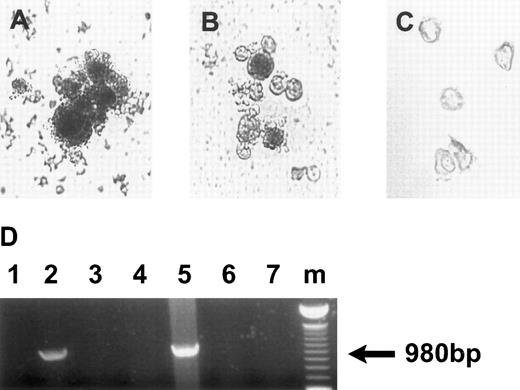
Analysis of HCMV persistence in the progeny derived from infected CD34+ cells.The recombinant virus Towne/lox2 was used to identify HCMV-infected cells in liquid cultures and within the colonies formed from virus-infected hematopoietic progenitor cells. Immediately after infection, cells were plated in methylcellulose, and 14 days later colonies were stained with X-gal; those containing Towne/lox2 appear blue (Fig 4A). Nine percent of the colonies derived from infected CD34+ cells contained β-gal activity. Infection with the control HCMV strains (not containing β-gal) showed no background staining (data not shown). LacZ expression was observed only in myelomonocytic colonies. Liquid cultures infected with recombinant strain were monitored for β-gal expression using X-gal staining 7, 14, 21, and 28 days after infection. LacZ activity was visualized in the progeny derived from CD34+ cells infected with the recombinant strain (Fig 4B). At 14 days postinfection, LacZ IE promoter expression was active in approximately 9% of cells in the culture. However, at 28 days postinfection, 40% of the cells were β-gal+. Progeny derived from mock-infected CD34+ cells and infected with other HCMV strains showed no background levels of LacZ activity by X-gal staining (Fig 4C). PCR analysis confirmed that LacZ expression correlated with the presence of β-gal DNA in the cells derived from CD34+ progenitors infected with recombinant HCMV strain (Fig 4D).
HCMV persistence in the progeny derived from infected CD34+ cells. (A) X-gal staining of the colonies derived from CD34+ cells infected with Towne/lox2 in methylcellulose cultures 14 days postinfection. Original magnification × 320. (B) X-gal staining of the progeny derived from CD34+ cells infected with Towne/lox2 in suspension cultures 28 days postinfection. Original magnification × 200. (C) Progeny derived from mock-infected CD34+ cells stained as in (A) and (B). Original magnification × 200. Cells from the colonies and suspension cultures were stained with X-gal (150 μg/mL) for 6 hours. (D) PCR detection of β-gal DNA in the progeny derived from CD34+ cells infected with Towne/lox2. PCR products were electrophoretically separated in 1% agarose gels stained with ethidium bromide. The arrow indicates a β-gal DNA PCR product of 900 bp. Lanes 1 through 3, DNA from colonies derived from infected and mock-infected CD34+ cells; lanes 4 through 6, DNA from suspension cultures derived from infected and mock-infected CD34+ cells. Lanes 1 and 4, DNA from the cultures infected with 95(2); lanes 2 and 5, DNA from the cultures infected with Towne/lox2; lanes 3 and 6, DNA from the cultures derived from mock-infected CD34+ progenitors; lane 7, PCR H2O control; lane m, 100-bp ladder (GIBCO/BRL). Primers used and predicted PCR products are listed in Table 1.
HCMV persistence in the progeny derived from infected CD34+ cells. (A) X-gal staining of the colonies derived from CD34+ cells infected with Towne/lox2 in methylcellulose cultures 14 days postinfection. Original magnification × 320. (B) X-gal staining of the progeny derived from CD34+ cells infected with Towne/lox2 in suspension cultures 28 days postinfection. Original magnification × 200. (C) Progeny derived from mock-infected CD34+ cells stained as in (A) and (B). Original magnification × 200. Cells from the colonies and suspension cultures were stained with X-gal (150 μg/mL) for 6 hours. (D) PCR detection of β-gal DNA in the progeny derived from CD34+ cells infected with Towne/lox2. PCR products were electrophoretically separated in 1% agarose gels stained with ethidium bromide. The arrow indicates a β-gal DNA PCR product of 900 bp. Lanes 1 through 3, DNA from colonies derived from infected and mock-infected CD34+ cells; lanes 4 through 6, DNA from suspension cultures derived from infected and mock-infected CD34+ cells. Lanes 1 and 4, DNA from the cultures infected with 95(2); lanes 2 and 5, DNA from the cultures infected with Towne/lox2; lanes 3 and 6, DNA from the cultures derived from mock-infected CD34+ progenitors; lane 7, PCR H2O control; lane m, 100-bp ladder (GIBCO/BRL). Primers used and predicted PCR products are listed in Table 1.
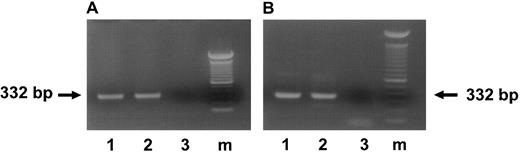
To determine how long HCMV DNA can persist in the infected cultures, both HCMV-infected and mock-infected cells harvested from suspension cultures and colonies derived from CD34+ cells progenitors were screened by nested PCR for the presence of HCMV DNA. Infected CD34+ progeny remained PCR-positive for HCMV DNA throughout the entire culture period (4 weeks; Fig 5A). As shown by nested PCR analysis, HCMV DNA was also present in the colonies derived from infected CD34+ progenitor cells (Fig 5B).
Presence of HCMV DNA in the progeny derived from HCMV-infected CD34+ BM cells. (A) Ethidium bromide-stained 1% agarose gel of PCR for HCMV DNA in suspension cultures derived from HCMV-infected CD34+ cells 28 days postinfection. (B) Ethidium bromide-stained 1% agarose gel of PCR for HCMV DNA in colonies derived from infected CD34+ cells 14 days postinfection. The arrows indicate the IE PCR product of 332 bp. Lane 1, DNA from the cultures infected with 95(2); lane 2, DNA from the cultures infected with Towne/lox2; lane 3, DNA from the mock-infected cultures; lane m, 100-bp ladder (GIBCO/BRL).
Presence of HCMV DNA in the progeny derived from HCMV-infected CD34+ BM cells. (A) Ethidium bromide-stained 1% agarose gel of PCR for HCMV DNA in suspension cultures derived from HCMV-infected CD34+ cells 28 days postinfection. (B) Ethidium bromide-stained 1% agarose gel of PCR for HCMV DNA in colonies derived from infected CD34+ cells 14 days postinfection. The arrows indicate the IE PCR product of 332 bp. Lane 1, DNA from the cultures infected with 95(2); lane 2, DNA from the cultures infected with Towne/lox2; lane 3, DNA from the mock-infected cultures; lane m, 100-bp ladder (GIBCO/BRL).
Analysis of HCMV gene expression in the progeny derived from infected CD34+ progenitor cells.Because we detected the presence of HCMV DNA in the infected CD34+ cells progeny for up to 4 weeks, in the following series of experiments we analyzed the changes in HCMV gene expression in infected cultures of CD34+-derived macrophages. RT-PCR with primers for major IE and MCP genes and immunochemistry with MoAb directed against the major IE and pp65 HCMV proteins were used.
CD34+-derived progeny expressed IE (IE1) and late (MCP) HCMV transcripts 3 and 4 weeks after infection (Fig 6A and B). Both spliced and unspliced IE transcripts were detected in cells infected with the HCMV clinical isolate (Fig 6A). However, spliced transcripts (218 bp) appeared to be present at a much lower concentration. In contrast, nested RT-PCR analysis of the CD34+ cells progeny infected with the HCMV recombinant laboratory strain expressed only unspliced species of IE mRNA. HCMV L transcripts were detected in the cells derived from infected CD34+ progenitors only late in the course of infection (3 to 4 weeks after infection), when all of the cultures showed the greatest degree of morphologic differentiation (Fig 6B). Infected CD34+-derived macrophages demonstrated the presence of IE and L antigens by immunostaining 4 weeks postinfection (Fig 7A through H). However, the signal was detected only in the adherent cell populations exhibiting morphology of highly differentiated macrophage cultures. The frequency and the intensity of the HCMV antigens expression varied with the virus strain (Table 3). The highest levels of IE HCMV antigen expression were observed in the cells infected with the clinical isolate (up to 50%). In contrast, only 28% of cells infected with Towne/lox2 exhibited expression of IE protein.
HCMV IE and L gene expression in the macrophages derived from infected CD34+ BM progenitor cells 28 days postinfection. (A) Ethidium bromide-stained 1% agarose gel of RT-PCR products of IE transcripts. The arrows indicate the RT-PCR products of unspliced IE transcripts of 332 bp and spliced IE transcript of 218 bp. Lane 1, RNA from the cultures infected with 95(2); lane 2, RNA from the cultures infected with Towne/lox2; lane 3, RNA from mock-infected cultures; lane 4, positive control of HCMV-infected fibroblasts displaying both the genomic size (unspliced; 332 bp) banding pattern and the RNA size (spliced; 218 bp) banding pattern; lane 5, RT-PCR H2O control. (B) Ethidium bromide stained 1% agarose gel of RT-PCR products of MCP transcripts. The arrows indicate RT-PCR product of unspliced MCP gene of 638 bp. Lane 1, RNA from the cultures infected with 95(2); lane 2, RNA from the cultures infected with Towne/lox2; lane 3, RNA from mock-infected cultures; lane m, 100-bp ladder (GIBCO/BRL). All RNA samples were treated with Rnase-free Dnase. Primers and predicted PCR products are listed in Table 1.
HCMV IE and L gene expression in the macrophages derived from infected CD34+ BM progenitor cells 28 days postinfection. (A) Ethidium bromide-stained 1% agarose gel of RT-PCR products of IE transcripts. The arrows indicate the RT-PCR products of unspliced IE transcripts of 332 bp and spliced IE transcript of 218 bp. Lane 1, RNA from the cultures infected with 95(2); lane 2, RNA from the cultures infected with Towne/lox2; lane 3, RNA from mock-infected cultures; lane 4, positive control of HCMV-infected fibroblasts displaying both the genomic size (unspliced; 332 bp) banding pattern and the RNA size (spliced; 218 bp) banding pattern; lane 5, RT-PCR H2O control. (B) Ethidium bromide stained 1% agarose gel of RT-PCR products of MCP transcripts. The arrows indicate RT-PCR product of unspliced MCP gene of 638 bp. Lane 1, RNA from the cultures infected with 95(2); lane 2, RNA from the cultures infected with Towne/lox2; lane 3, RNA from mock-infected cultures; lane m, 100-bp ladder (GIBCO/BRL). All RNA samples were treated with Rnase-free Dnase. Primers and predicted PCR products are listed in Table 1.
HCMV IE and pp65 expression in macrophages derived from infected CD34+ progenitors 28 days postinfection. Cells were counterstained with Methylene Green. (A and B) Cells incubated with MoAb directed against IE protein. (E through H) Cells incubated with MoAb directed against pp65. (A and E) Macrophages derived from CD34+ cells infected with 95(2). (B and F ) Macrophages derived from CD34+ cells infected with Towne/lox2. (C and G) Macrophages derived from mock/infected CD34+ cells. (D and H) HCMV-infected HEL cells, as a positive control. Original magnification × 400.
HCMV IE and pp65 expression in macrophages derived from infected CD34+ progenitors 28 days postinfection. Cells were counterstained with Methylene Green. (A and B) Cells incubated with MoAb directed against IE protein. (E through H) Cells incubated with MoAb directed against pp65. (A and E) Macrophages derived from CD34+ cells infected with 95(2). (B and F ) Macrophages derived from CD34+ cells infected with Towne/lox2. (C and G) Macrophages derived from mock/infected CD34+ cells. (D and H) HCMV-infected HEL cells, as a positive control. Original magnification × 400.
Virus production in the cultures of monocytes and macrophages derived from HCMV-infected progenitor cells.Expression of L HCMV gene products (mRNA and protein) in the infected CD34+-derived progeny suggested that the virus productively infected these cells. To confirm this possibility, a routine plaque assay with human fibroblast cells was performed. Supernatants from infected and mock-infected CD34+ cultures were collected at 1, 2, 3, and 4 weeks postinfection and applied onto HEL cells. Incubation of human fibroblasts with the supernatants from myelomonocytic cells for an additional 2 weeks resulted in typical HCMV foci only if they were collected from the cultures initially infected with clinical isolate 95(2) at least 4 weeks after infection (Table 4). Nevertheless, the titer of the virus recovered from these supernatants was low (2.0 × 101 PFU/mL). Comparable data were obtained when freeze-thawed lysates were examined; by day 28 after infection, 1.6 × 101 PFU/mL of cellular lysate was recovered from the BM cultures infected with the clinical isolate.
In contrast, no virus was recovered from the supernatants and freeze-thawed lysates of the cells infected with laboratory strain or from the supernatants collected early in the course of infection (1, 2, and 3 weeks postinfection), indicating that the productive HCMV life cycle can be completed only in terminally differentiated macrophage cultures derived from CD34+ BM progenitors infected with clinical isolates but not the laboratory adapted strains.
As suspension cultures of CD34+ cells expanded, cell numbers accumulated from 107 to 108 cells per culture. The number of the infected cells increased parallel to the BM cells proliferation. As determined by direct count of cells, in CD34+ cultures cell number increased 100- to 300-fold over a period of 4 weeks. Per estimate, the number of infected cells in the infected cultures increased proportionally, resulting in 2 × 107 infected cells produced by 4 × 105 initial CD34+ progenitors inoculated with HCMV clinical isolate or laboratory strain.
DISCUSSION
Although significant progress has been made in prevention of HCMV transmission, the pathophysiology of HCMV persistence and latency as well as its reactivation in seropositive hosts is unclear. Major contributions to the understanding of HCMV life cycle have been derived from the investigation performed using the cultured fibroblasts that support lytic HCMV infection. However, based on the clinical experience demonstrating that HCMV persists in PBM, it was suggested that these cells may be involved in virus spread.2,3 23 We present here a model of HCMV infection in hematopoietic cells.
Consistent with previous reports38,39,45,46 using PCR analyses and HCMV recombinant strain expressing LacZ, we show that hematopoietic progenitor cells can be infected by HCMV in vitro. Our results suggest that HCMV-infected BM progenitor cells may retain their ability to proliferate and differentiate. However, in suspension cultures designed to promote monocytic differentiation and proliferation, growth of the CD34+ progenitors was significantly inhibited by a clinical isolate of HCMV, but not the laboratory strain. Surprisingly, the laboratory strain enhanced the production of mononuclear phagocytes in suspension cultures, whereas no difference was seen in colony formation assayed in methylcellulose cultures. These results suggest that proliferation rate of an individual progenitor cell was enhanced. A possible explanation for the differential effect of clinical isolates versus the Towne/lox2 strain is the nearly 20-kb deletion present in the genome of the Towne laboratory strain. These deleted genes may have a strong suppressive effect on hematopoietic cell proliferation. Similar differences in biologic properties between laboratory strains and low passaged clinical isolates, including the ability to modulate hematopoiesis, have been reported by other investigators.31,35,38 In addition, we have shown that HCMV is more potent in suppressing the formation of myelomonocytic colonies than colonies of erythroid lineage, providing additional evidence for the preferential infection of myelomonocytic progenitor cells by HCMV. This observation is supported by our studies showing that, in cells derived from CD34+ progenitor cells infected with a recombinant strain, LacZ activity was restricted to myelomonocytic colonies and by the previous studies in which PBM have been shown to be the major site of HCMV latency in healthy seropositive individuals.2,3,23 44
Our current report shows that HCMV DNA can persist in suspension cultures initiated by CD34+ BM cells infected with HCMV for at least 4 weeks after infection. The presence of HCMV DNA and the inability to detect viral antigens (IE or L) after 7 days of infection extended our previous observations42 showing limited gene expression was present in CD34+ cells in the first days of infection with HCMV. Our data strongly suggest that HCMV infection of early hematopoietic cells is not lytic but results in the persistence of HCMV DNA in the initially infected cells or their progeny. Because no virus was detected in the supernatants of HCMV-infected CD34+ cultures, we conclude that HCMV DNA spread through the expansion of the number of HCMV genome-carrying cells generated by the proliferation of infected precursors. Because infected BM progenitors maintained their ability to proliferate and differentiate along the monocytic lineage, the number of infected cells increased in parallel with the expansion of the BM suspension cultures as determined using our HCMV recombinant strain and immunostaining with MoAbs against IE and L HCMV proteins. These results suggest that the HCMV genome is able to replicate in BM progenitors along with the cell proliferation without lytic gene expression. Such a mechanism of persistence in hematopoietic cells would allow the virus to spread widely from a single infected BM progenitor cell to a numerous progeny and persist for a long period of time (as long as a lifetime of the host) without elimination by the host immune response.
In agreement with previous reports,26-29,43,46,47 expression of L genes products (mRNA and protein) occurred only late in the course of infection of mononuclear phagocytes (3 to 4 weeks postinfection) and correlated with the complete differentiation of the macrophages derived from the infected BM progenitor cells. Consistent with this observation in our study, infectious virus could be recovered only from the supernatants of cultured CD34+-derived macrophages infected with a clinical isolate. The titer of the virus recovered from these supernatants was extremely low, consistent with the delayed kinetics of the virus reactivation from latency. In addition, in previous reports, HCMV infection of monocyte-derived macrophages has been characterized as predominantly cell associated.23,42 This finding supports the theory that a productive infection in infected CD34+ progeny is possible but only after differentiation of these cells into macrophages. Absence of infectious virus in the supernatants of the CD34+ progeny infected with a laboratory strain is in agreement with an earlier report of an atypical splicing pattern in granulocyte-macrophage progenitors.43 In vivo, when the pressure exerted by the immune system declines (eg, after immunosuppressive therapy), virus released from latently infected macrophages may be a source of HCMV dissemination and clinical HCMV disease.
It is unlikely that the observed lytic HCMV gene expression and recovery of the infectious virus from the supernatants of the macrophages derived from infected CD34+ cells was caused by the initial contamination of the CD34+ cells cultures with peripheral blood monocytes/macrophages. First, the purity of the isolated CD34+ cells using biotin-avidin affinity column was 85% to 95%, as confirmed by the fluorescence-activated cell sorting analysis. Second, because the infected BM cultures expanded 100- to 300-fold over a period of 4 weeks and were passed at least twice every week, the possibility of the 4-week BM cultures being contaminated with monocytes/macrophages that persisted this long is extremely low. Similarly, the presence of HCMV DNA in BM cultures and infectious virus in the supernatants of the infected CD34+-derived macrophages was not due to the contamination with the virus inoculum, because cells were thoroughly washed free of the virus after adsorption and infectious virus was not detected in the supernatants of the BM cultures earlier than 4 weeks after infection or in the supernatants of cultures infected with the laboratory strain. In addition, we would like to point out that an unusual localization of the IE antigen was observed in infected CD34+-derived macrophages, which is normally located within the nuclei of the infected cells. Only a portion of the staining was located in the nuclei and the majority of the protein was found in the cytoplasm, mostly in the perinuclear area. This abnormal IE protein localization seen in infected BM progenitor-derived macrophages was not an artifact of the immunostaining procedure, because IE expression was seen in the nuclei of the infected HEL cells and none was found in the cytoplasm. The mechanism underlying the observed unusual IE protein localization was not further investigated and beyond the scope of this study. However, one possible explanation for our findings could be that the atypical splicing pattern of mRNA detected by RT-PCR in infected CD34+-derived monocyte/macrophages could be responsible for the aberrant localization of IE signal. The presence of the spliced message appeared not to be an artifact, because they were reproducibly present in cultures infected with several clinical isolates and were consistent with the splicing pattern observed in infected myeloid progenitor cells.43 This finding may possibly be related to the new lytic nature of HCMV infection in hematopoietic cells.
In summary, we propose a model of persistent HCMV infection in hematopoietic cells. According to this model, BM hematopoietic progenitor cells can acquire HCMV early in their development in BM. HCMV DNA persists in these cells and replicates along with BM progenitors for an extensive time period without lytic gene expression. Because the virus can infect early clonogenic progenitors without eliminating their ability to proliferate and differentiate, HCMV can create a pool of infected BM progenitor cells that can serve as a reservoir of latent virus. The mechanism(s) underlying the observed effect of cell differentiation on HCMV reactivation is unclear. It is possible that expression of a specific intracellular factor stimulated by the cell differentiation induces HCMV lytic gene expression. Another alternative is an autocrine induction of HCMV lytic gene expression by one or several cytokines produced by the differentiated macrophages.
ACKNOWLEDGMENT
The authors thank Dr W.L. Drew for providing the HCMV clinical isolates.
Supported by NIH Grants No. AI 36418 and HL 48503.
Address reprint requests to S. St Jeor, PhD, University of Nevada, Reno, Microbiology Department, Medical School, Howard Bldg, Room 208, Reno, NV 89557.