To the Editor:
Several reports have established an impotant role for Fas/Fas ligand (FasL) interaction in mediating programmed cell death of CD4+ lymphocytes in human immunodeficiency virus (HIV)-infected subjects.1,2 Soluble forms of the Fas receptor and its ligand have also been detected in supernatants of cultured cells or in human serum.3,4 Elevated serum levels of soluble Fas were found to occur during HIV infection; however, no correlation could be established between serum Fas levels and stage of infection or progression to disease.5 Nevertheless, the enhanced release of soluble Fas was suggested to be a host-defense mechanism against HIV infection and aberrant apoptosis induction. On the other hand, soluble FasL was found to induce apoptosis in Fas-positive lymphocytes,4,6 and increased serum levels were reported in certain cancer patients and were associated with systemic tissue damage.7 In addition, HIV infection of CD4+ T cells8 and macrophages9 was found to upregulate FasL expression, leading to enhanced lymphocyte apoptosis. Therefore, to gain further insight into the role of FasL in the T-cell depletion during HIV infection, we have analyzed serum levels of FasL from HIV-infected subjects during the asyptomatic or the symptomatic/disease stage and compared the levels with those in sera of matched healthy controls.
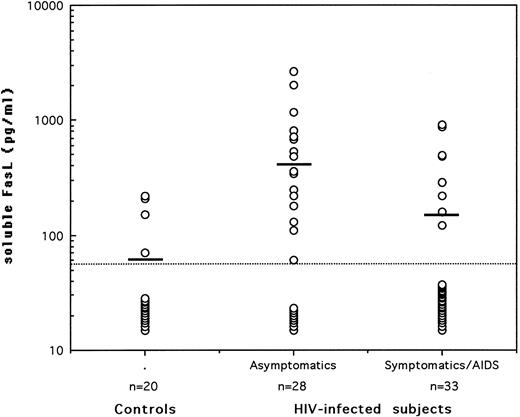
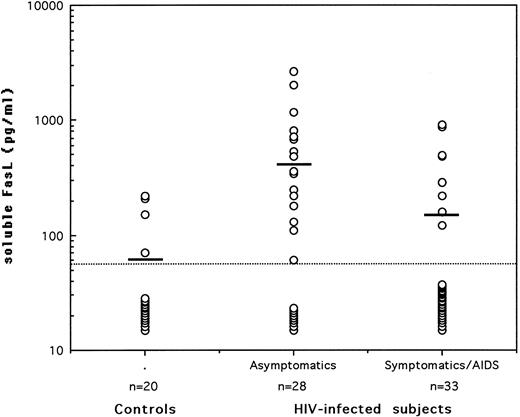
Serum levels of soluble FasL in asymptomatic HIV-seropositives, symptomatic/AIDS patients, and normal healthy controls. Soluble FasL was quantitated by sandwich ELISA using 2 hamster monoclonal antibodies. The dotted line represents the limit of detection in the assay and the horizontal bars indicate the arithmetic mean of the data.
Serum levels of soluble FasL in asymptomatic HIV-seropositives, symptomatic/AIDS patients, and normal healthy controls. Soluble FasL was quantitated by sandwich ELISA using 2 hamster monoclonal antibodies. The dotted line represents the limit of detection in the assay and the horizontal bars indicate the arithmetic mean of the data.
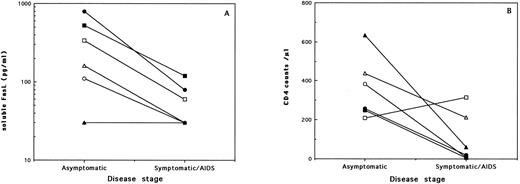
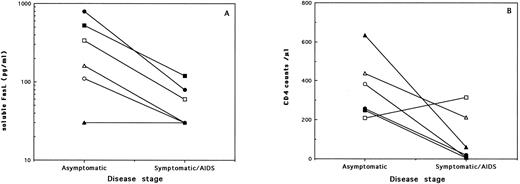
(A) Serum levels of soluble FasL and (B) CD4 counts from 6 HIV-infected individuals evaluated at different stages of the disease. Each symbol represents the values from one subject examined during the asymptomatic period and then 2 to 7 years later, during the symptomatic/AIDS stage. Statistically significant decrease in serum FasL (P = .0431; Wilcoxon matched-pairs test) and CD4 counts (P = .0464) was noted with disease progression.
(A) Serum levels of soluble FasL and (B) CD4 counts from 6 HIV-infected individuals evaluated at different stages of the disease. Each symbol represents the values from one subject examined during the asymptomatic period and then 2 to 7 years later, during the symptomatic/AIDS stage. Statistically significant decrease in serum FasL (P = .0431; Wilcoxon matched-pairs test) and CD4 counts (P = .0464) was noted with disease progression.
The quantification of serum FasL was performed using a sandwich enzyme-linked immunosorbent assay (ELISA) based on the use of 2 hamster monoclonal antibodies to FasL.7 The minimum detectable level of FasL was reproducibly found to be 60 pg/mL using recombinant FasL protein as a standard diluted in normal (FasL-negative) human serum. For statistical analysis, serum samples that showed no detectable FasL were attributed a level of 30 pg/mL, equivalent to half of the lowest detectable concentration. The subjects tested were 20 HIV-seronegative healthy controls, 28 asymptomatic HIV-seropositives (grade A according to the revised CDC classification), and 33 symptomatic/acquired immunodeficiency syndrome (AIDS) patients (18 with grade B and 15 with grade C). The asymptomatic patients had a mean CD4 count of 536/μL; none of them presented with opportunistic infections and 11 of the 28 (39%) were receiving antiretroviral therapy. On the other hand, the symptomatic/AIDS patients had a mean CD4 count of 333/μL; only 4 of them (12%) had associated opportunistic infections at the time serum samples were taken and 79% were receiving antiretroviral drugs. Plasma viral loads were available on samples from only 12 asymptomatics and 21 patients. No significant differences in viral loads (P > .05; Mann-Whitney U rank test) were detected among the 2 groups, although viral copies less than 2 × 103/mL were observed in 75% of the asymptomatic patients and only in 48% of the symptomatic/AIDS cases.
The levels of soluble FasL in sera of all tested subjects are shown in Fig 1. Detectable levels were observed in sera of 30% of either healthy controls or symptomatic/AIDS patients and in 68% of HIV-seropositive asymptomatics (P = .0104 v controls and P = .0035 v patients; Fisher's exact test). The mean ± standard error of the mean of serum FasL levels (in picograms per milliliter) were 60 ± 10 in healthy subjects, 150 ± 40 in patients (P > .05; Mann-Whitney U rank test), and 410 ± 120 in the asymptomatic patients (P = .0025 and P = .0111 v controls and patients, respectively). No positive or negative correlation could be found in either of the HIV-infected groups between serum FasL levels on one hand and CD4 counts, viral load, or presence of antiretroviral therapy on the other hand. Nevertheless, a significant decrease in CD4 counts (P = .0042) accompanied with a significant decrease in serum FasL levels was associated with progression to disease. None of the tested markers was significantly different between grade B symptomatics and grade C AIDS patients.
To verify the correlation between the decrease in serum FasL and disease progression, we have analyzed serum samples obtained from the same patients over a period of 1 to 7 years. Samples from 6 asymptomatic patients who had progressed to disease were available. Results on the changes in serum FasL levels and CD4 counts are shown in Fig 2. It was evident that, among all 5 of the subjects who presented with detectable soluble FasL in their sera during the asymptomatic period, 2 became negative and 3 showed highly reduced levels 2 to 5 years later, during the disease stage. One subject who was negative for serum FasL during the asymptomatic phase remained negative as disease progressed after a period of 7 years. Interestingly, we also had available serum samples taken at two time points from 9 asymptomatic patients who did not show signs of disease progression over a period of 1 to 7 years. Two of these subjects initially tested as negative for serum FasL remained negative when examined 1 or 4 years later. However, the other 7 asymptomatic patients who presented with positive serum FasL levels (mean, 207 pg/mL) all remained positive, with comparable or even higher levels (mean, 357 pg/mL) when examined again within 1 to 7 years (mean, 4 years) during the asymptomatic phase. It is relevant to indicate that this stability in serum FasL levels during the asymptomatic stage occurred in the absence of comparable stability in CD4 counts, in which a 47% mean decrease (range, 8% to 80%) was noted between the same two time points.
Increased FasL mRNA accumulation has been reported in HIV-infected subjects and was found to correlate with disease progression.10 More recently, soluble and membrane-bound forms of FasL were found to be produced in greater amounts in cell cultures and in plasma from HIV-infected persons than from healthy controls.11 Our findings of elevated serum FasL only during the asymptomatic stage could point to a defective process of metalloproteinase-mediated release of FasL12 in symptomatic/AIDS patients. Alternatively, because enhanced expression and secretion of FasL has been observed from Th-1 cells but not from Th-2 cells,13 it is possible that the reduced levels in the disease stage stems from a decrease in T-cell numbers and more specifically in the Th-1 population. In any case, the maintained elevation of serum FasL in asymptomatic patients could still be a useful marker for absence of disease progression. Future studies are warranted to evaluate this phenomenon in the context of apoptosis levels, to study the differential regulation of Fas and FasL secretion, and to further characterize the role of soluble FasL in the pathogenesis of HIV disease.
ACKNOWLEDGMENT
This work was supported by research grants from Agence Nationale de Recherches sur le SIDA and the Fondation pour la Recherche Medicale in France.