ABOUT 40 YEARS ago two forms of chronic myelocytic leukemia (CML) were recognized in children. One had the typical features of CML of adulthood and usually appeared in children older than 4 years; the other affected younger children and presented as a myelomonocytic proliferation associated with hemorrhage, infection, lymphadenopathy, and skin rash. The prognosis of the latter group of patients was invariably poor.1-3 In subsequent attempts to identify clinico-biological features that would further discriminate between these leukemias, Reisman and Trujillo4 found the absence of the Philadelphia (Ph) chromosome to be a hallmark of the juvenile-type entity (JCML). Signs of disturbed erythropoiesis were also reported to be typical of JCML, including low levels of hemoglobin (Hb) A2 and erythrocyte carbonic anhydrase,5 together with a marked increase of HbF,6 and glucose-6-phosphate dehydrogenase activity.7 Maurer and others concluded that JCML is accompanied by a reversion to fetal-like erythropoiesis.8-10 The observation of chromosomal translocations in leukemic bone marrow cells of different lineages suggested that the pathologic process might involve a multipotent stem cell.7 11 We review here the salient clinical and biological features of this disease, emphasizing recent advances in its treatment and in understanding its pathological origin.
CLASSIFICATION
The classification of chronic childhood myeloproliferative disorders remains controversial. Although the proposed categories of the French-American-British (FAB) system do not always correspond to the clinical features of such cases,12 they still constitute the standard for comparative evaluation of different series of patients. Patients who present with peripheral blood monocytosis and bone marrow dysplasia (often in association with monosomy 7) with a morphological picture of chronic myelomonocytic leukemia (CMML) in the absence of the t(9; 22) translocation are generally considered to have the juvenile form of CML. However, in different reports, patients sharing most of the above features are variously described as having JCML,7,8,10,13-15 monosomy 7 syndrome,16,17 or CMML in accord with the FAB system.12,18,19 Using features such as cytogenetic abnormalities and pretreatment HbF level, Passmore et al20 recently reclassified their cases as (1) JCML if there was CMML cellular morphology and raised HbF (>10%), and no monosomy 7; or (2) infantile monosomy 7 syndrome if the presenting age was less than 4 years, with any type of myelodysplasia and monosomy 7. All other patients, including older children with monosomy 7, were classified according to FAB criteria. The revised classification led the authors to propose a new prognostic scoring system.20 Although the aim of providing an improved system of risk assessment is laudable, the utility of the scoring system proposed by Passmore et al20 may be compromised by the criteria they used to reclassify cases of childhood myelodysplastic syndrome (MDS). In particular, some categories, such as the monosomy 7 syndrome, are widely debated or not accepted by some investigators.18 19 Thus, the uncertainty of diagnostic criteria in this group of disorders can be expected to limit the utility of the proposed scoring system.
In an effort to resolve most of the above described discrepancies, the International Juvenile Myelomonocytic Leukemia Working Group has recently proposed the term juvenile myelomonocytic leukemia (JMML; R. Castleberry, personal communication, December 1996), which has been accepted and widely used in some recent publications.21-23 It will be used throughout the remainder of this review.
EPIDEMIOLOGY
The exact incidence of JMML is not yet known. In the only population-based study reported to date,18,24 JMML accounted for 18% of all cases of myelodysplastic syndrome in children less than 15 years old and 1.62% of all hematologic malignancies (0.61 new cases per year per million children at risk). The disease predominates in children younger than 4 years with 40% of cases occurring before the age of 1 year and 60% before the age of 2; however, 26% of patients with JMML are 3 years of age or older. Boys are affected more often than girls (M/F ratio, 2.5). Associated conditions include neurofibromatosis type 1 (NF1)25-27 in 7%, and a variety of occasional clinical abnormalities in an additional 9% of cases. At least three pairs of affected twins have been described.15,20,28 In another study, an identical twin was healthy at the time his brother presented with JMML and remained free of leukemia during preparation of the written report.29
CLINICAL AND LABORATORY FEATURES
The onset of JMML is often heralded by acute or subacute symptoms of a recent infection. Table 1 summarizes clinical and laboratory features of patients variably referred to as juvenile chronic granulocytic leukemia (JCGL),11,29 JCML,13,15,20,30-35,36,37-42 CMML20,28,43 or monosomy 7.20 40 As shown, more than half of the patients have fever and signs of respiratory involvement, such as pharyngo-tonsillitis or bronchitis; pulmonary infection is infrequent. Bleeding symptoms are observed in about 50% of cases. Prominent findings are those common to most proliferative diseases: enlarged spleen, hepatomegaly, and lymphadenopathy. Skin involvement is apparent in about one half of the patients, with maculo-papular rash (often starting from the face) and xanthomas, or cafe-au-lait spots, seen in patients with NF1.
Presenting Features of Children With JMML
| Characteristic . | Category . | No. . | % . |
|---|---|---|---|
| Clinical | |||
| Sex | Male | 146 | 71 |
| Female | 60 | 29 | |
| Age | <1 yr | 72 | 40 |
| 1 to <2 yr | 36 | 20 | |
| 2 to <3 yr | 25 | 14 | |
| ≥3 yr | 46 | 26 | |
| Hepatomegaly | 165 | 79 | |
| Splenomegaly | 206 | 97 | |
| Lymphadenopathy | 89 | 52 | |
| Skin involvement | 78 | 48 | |
| Associated conditions | NF1 | 14 | 7 |
| Other conditions | 17 | 9 | |
| Laboratory | |||
| Leukocyte count (×109/L) | <50 | 144 | 70 |
| 50-99 | 39 | 22 | |
| ≥100 | 14 | 8 | |
| Monocyte count (×109/L) | <5 | 67 | 35 |
| 5-9.9 | 45 | 32 | |
| ≥10 | 46 | 33 | |
| Percentage of blasts in peripheral blood | 0-2 | 92 | 56 |
| >2 | 73 | 44 | |
| Hb level (g/dL) | >8-12 | 100 | 66 |
| <8 | 53 | 34 | |
| HbF level (%) | 0-9 | 71 | 38 |
| ≥10 | 117 | 62 | |
| Platelets count (×109/L) | ≥100 | 40 | 23 |
| 99-50 | 54 | 30 | |
| <50,000 | 80 | 47 | |
| Bone marrow blasts (%) | 0-9 | 67 | 64 |
| 10-20 | 27 | 26 | |
| >20 | 12 | 10 | |
| Alkaline phosphatase score | Low | 47 | 41 |
| Cytogenetic analysis | Normal | 132 | 68 |
| Monosomy 7 | 31 | 16 | |
| Other abnormalities | 32 | 16 |
| Characteristic . | Category . | No. . | % . |
|---|---|---|---|
| Clinical | |||
| Sex | Male | 146 | 71 |
| Female | 60 | 29 | |
| Age | <1 yr | 72 | 40 |
| 1 to <2 yr | 36 | 20 | |
| 2 to <3 yr | 25 | 14 | |
| ≥3 yr | 46 | 26 | |
| Hepatomegaly | 165 | 79 | |
| Splenomegaly | 206 | 97 | |
| Lymphadenopathy | 89 | 52 | |
| Skin involvement | 78 | 48 | |
| Associated conditions | NF1 | 14 | 7 |
| Other conditions | 17 | 9 | |
| Laboratory | |||
| Leukocyte count (×109/L) | <50 | 144 | 70 |
| 50-99 | 39 | 22 | |
| ≥100 | 14 | 8 | |
| Monocyte count (×109/L) | <5 | 67 | 35 |
| 5-9.9 | 45 | 32 | |
| ≥10 | 46 | 33 | |
| Percentage of blasts in peripheral blood | 0-2 | 92 | 56 |
| >2 | 73 | 44 | |
| Hb level (g/dL) | >8-12 | 100 | 66 |
| <8 | 53 | 34 | |
| HbF level (%) | 0-9 | 71 | 38 |
| ≥10 | 117 | 62 | |
| Platelets count (×109/L) | ≥100 | 40 | 23 |
| 99-50 | 54 | 30 | |
| <50,000 | 80 | 47 | |
| Bone marrow blasts (%) | 0-9 | 67 | 64 |
| 10-20 | 27 | 26 | |
| >20 | 12 | 10 | |
| Alkaline phosphatase score | Low | 47 | 41 |
| Cytogenetic analysis | Normal | 132 | 68 |
| Monosomy 7 | 31 | 16 | |
| Other abnormalities | 32 | 16 |
Data from 11,13,15,20,29-43. Certain features are not reported for all patients.
Leukocyte counts tend to remain less than 50 × 109/L; in fact, fewer than 8% of cases have counts greater than 100 × 109/L. Monocytosis exceeding 5 × 109/L with circulating immature myeloid cells, erythroblasts, and a few blasts (<3% in 56% cases) are typical findings. A decreased leukocyte alkaline phosphatase score is not characteristic, occurring in less than 50% of the patients. The Hb level is usually low and is accompanied by erythrocyte abnormalities and low serum iron concentrations. An altered Hb pattern affords a readily accessible and reliable marker of JMML; at diagnosis the HbA2 concentration is reduced, whereas HbF level greater than 10% is found in two-thirds of patients. Thrombocytopenia is common and often severe. Elevated levels of serum vitamin B12 are frequently observed and appear related to active protein synthesis by granulocytes.44 Polyclonal hypergammaglobulinemia is also common, but its significance remains unclear.45 Increased serum levels of lysozyme are reported in two thirds of the patients in association with a higher number of immature circulating granulocytic precursors.19 Autoantibodies with different reactivities (antinuclear type or positive Coombs test) and no apparent clinical relevance have been observed in fewer than one fourth of the patients.19
An analysis of peripheral blood usually provides more reliable diagnostic information than does an examination of the bone marrow smear. Typically, marrow cellularity is increased, with the vast majority of cells belonging to the myeloid series in all stages of maturation. Monocytes account for 5% to 10% of all myeloid cells, with the blast cell population far below the level seen in acute leukemia. Follow-up marrow evaluations rarely yield informative results. In contrast to other types of childhood leukemia, JMML has few discernible constitutional or clonal chromosome abnormalities, partly explaining the slow progress in understanding the pathobiology of this disease. In one study, 68% of the reported cases of JMML lacked cytogenetic abnormalities, the remainder having either dissimilar lesions (16%) or monosomy 7 (16%).46-48
More recently in a retrospective analysis of 110 patients defined by entry criteria and treated in five European countries,19 the European Working Group on Myelodysplastic Syndromes in childhood (EWOG-MDS) found a constellation of features to have diagnostic relevance: hepatosplenomegaly in greater than 90%, lymphadenopathy in 76%, pallor in 64%, fever in 54%, skin rash in 36%, absence of the 9; 22 translocation, bone marrow blasts less than 20%, and peripheral blood monocytosis greater than 1 cell × 109/L. Additional criteria required for definite diagnosis included at least two of the following: spontaneous in vitro growth of granulocyte-macrophage progenitors (CFU-GM), HbF elevated for age, peripheral blood myeloid precursors, leukocyte count greater than 10 × 109/L, and chromosomal abnormalities.
NATURAL COURSE OF THE DISEASE AND PROGNOSTIC FACTORS
The observation of patients who either were not treated or did not respond to treatment has provided useful information on the natural history of JMML. Approximately one third of the patients present with a rapidly progressive disease in which cachexia, organomegaly, and complications of marrow failure may lead to early death, whether or not treatment is instigated. About another third of the patients show a more indolent disease course characterized by clinical improvement with partial or even complete normalization of the blood count after minimal or no treatment. Such patients may enjoy a stable clinical remission, even when there is persistence of a variable pattern of disease markers, including splenomegaly, moderate leukocytosis, or monocytosis. During this true chronic phase of the disease, they may occasionally experience reactivation of their leukemia, sometimes in association with infectious episodes. Because these episodes are often self-limiting, introduction of chemotherapy at this time may produce what appears to be a clinical response. The ultimate outcome of leukemia is difficult to predict, but a significant proportion of patients eventually develop massive disease reactivation evolving into a rapid progression, often referred to as blastic crisis.15,29 49 The remaining patients have an intermediate prognosis.
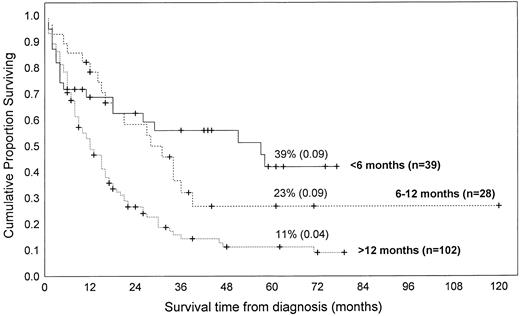
Because the course of JMML is highly variable and the therapeutic choices wide, some investigators have attempted to identify factors that predict outcome or at least time to progression. The major role in defining prognosis seems to be played by age at the time of disease onset. Most published series support the generalization that children who develop the disease when younger than 1 or 2 years have a longer survival.15,20,30 50 In our review, infants younger than 1 year of age had a significantly higher probability of long-term survival than did older patients; there was no significant difference between infants presenting at the age of less than 6 months or 6 to 12 months. These relationships were valid whether patients treated with bone marrow transplantation were excluded (Fig 1) or included (not shown) in the analysis. This analysis should be regarded with caution both because we can only refer to published data and because data were taken from sources without uniform entry criteria and do not represent randomized clinical trials.
Kaplan-Meier survival estimates of 177 children with JMML according to age at the diagnosis. The curves were generated by pooling the data from the following references, by including only cases for which both informations (ie, age at diagnosis and duration of survival) were available.8,11,13,27,28,30-37,41,47,82,85,97-99,101 103
Kaplan-Meier survival estimates of 177 children with JMML according to age at the diagnosis. The curves were generated by pooling the data from the following references, by including only cases for which both informations (ie, age at diagnosis and duration of survival) were available.8,11,13,27,28,30-37,41,47,82,85,97-99,101 103
Among the proposed unfavorable risk factors are a low platelet count and an increased HbF level15,20 (Table 2). In a recent review of their single-center series of childhood MDS patients, Passmore et al20 advocated a pediatric scoring system based on HbF level, platelet count, and cytogenetic results. The so-called FPC score was predictive of a good prognosis (60% survival at 5 years) when HbF was greater than 10%, platelets greater than 40 × 109/L, and clonal cytogenetic abnormalities were absent.
Unfavorable Prognostic Factors in JMML
| Feature . | Study (reference) . | Feature . | Study (reference) . |
|---|---|---|---|
| Age (mo) | HBF level | ||
| >48 | Niemeyer19 | Increased | Castro15 |
| >24 | Castro,15 Passmore20 | >15% | Niemeyer19 |
| >12 | Aricò,30 Pui50 | >10% | Owen,29 Passmore20 |
| >6 | Owen29 | Thrombocytopenia (×109/L) | |
| Male sex | Owen29 | <100 | Castro15 |
| <50 | Aricò30 | ||
| Bleeding | Castro,15 Owen29 | <40 | Passmore20 |
| Niemeyer19 | <33 | Niemeyer19 | |
| Hepatomegaly (>3 cm) | Castro15 | Blasts in peripheral blood | |
| ≥2 × 109/L | Castro15 | ||
| Hb level (<10 g/dL) | Niemeyer19 | >4% | Niemeyer19 |
| Reticulocytes (<10%) | Niemeyer19 | Normoblasts in peripheral blood (>1 × 109/L) | Castro15 |
| FPC score (≥2)* | Passmore20 | Blasts in the BM myeloid compartment (>5%) | Niemeyer19 |
| Feature . | Study (reference) . | Feature . | Study (reference) . |
|---|---|---|---|
| Age (mo) | HBF level | ||
| >48 | Niemeyer19 | Increased | Castro15 |
| >24 | Castro,15 Passmore20 | >15% | Niemeyer19 |
| >12 | Aricò,30 Pui50 | >10% | Owen,29 Passmore20 |
| >6 | Owen29 | Thrombocytopenia (×109/L) | |
| Male sex | Owen29 | <100 | Castro15 |
| <50 | Aricò30 | ||
| Bleeding | Castro,15 Owen29 | <40 | Passmore20 |
| Niemeyer19 | <33 | Niemeyer19 | |
| Hepatomegaly (>3 cm) | Castro15 | Blasts in peripheral blood | |
| ≥2 × 109/L | Castro15 | ||
| Hb level (<10 g/dL) | Niemeyer19 | >4% | Niemeyer19 |
| Reticulocytes (<10%) | Niemeyer19 | Normoblasts in peripheral blood (>1 × 109/L) | Castro15 |
| FPC score (≥2)* | Passmore20 | Blasts in the BM myeloid compartment (>5%) | Niemeyer19 |
Relevant in all cases of childhood MDS, including JMML.
PATHOBIOLOGY
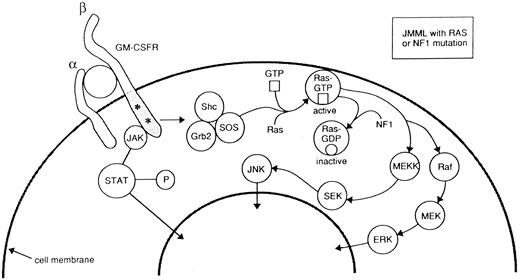
Two consistent abnormalities have been shown by in vitro studies of JMML patients: the exuberant spontaneous growth of colony forming units-granulocyte/macrophage (CFU-GM) in the absence of exogenous growth factors,51-54 and the impaired growth of normal hemopoietic progenitors. Spontaneous cell growth has been ascribed to the peculiar hypersensitivity of the JMML bone marrow or peripheral blood progenitors to very low levels of cytokines and growth factors probably produced by adherent cells.55 Removal of monocytes by adherence to plastic before culture of bone marrow or peripheral blood progenitor cells abrogates spontaneous growth.55 The observed hypersensitivity response appears to be quite selective, as it occurs in tests with GM-CSF, but not other growth factors regulating early steps of myelopoiesis, such as inteleukin-3 (IL-3) or granulocyte colony-stimulating factor (G-CSF ).52 To date, binding studies and molecular analysis of the GM-CSF receptor have shown no abnormality,56 although several provocative clues have emerged (Fig 2). During neoplastic transformation of myeloid progenitors, RAS genes often acquire activating point mutations that lead to elevated levels of Ras-GTP, resulting in constitutive activation of this common signal transduction pathway.57 In this regard, mutated RAS genes have been found in 30% of cases of JMML.34,58-60 In one series most of the patients with mutated RAS were in the older age group with poor prognosis.34 Loss of the normal NF1 allele is a common finding in JMML cells from patients with NF1.61,62 As a tumor suppressor, the NF1 protein acts as GTPase, which in turn downregulates Ras-GTP. Primary leukemic cells from children with NF1 show a selective decrease in NF1-like GTPase activating protein (GAP) activity for RAS, but retain normal cellular GAP activity. Leukemic cells also show an elevated percentage of Ras in the GTP-bound conformation.63 These genetic and biochemical data from studies of primary JMML cells strongly support the hypothesis that inactivation of NF1 with consequent Ras deregulation plays an important role in leukemogenesis in children with NF1 who develop JMML. This idea is reinforced by a number of observations in mice with a targeted disruption of Nf1. First, heterozygous Nf1 mice are predisposed to myeloid leukemia and these leukemias delete the wild-type Nf1 allele.64 Second, fetal hematopoietic cells from embryos with homozygous inactivation of Nf1 show a pattern of selective in vitro GM-CSF hypersensitivity that is reminiscent of that seen in JMML.62,63 Finally, transplanting these Nf1 −/− fetal liver cells into irradiated recipients consistently induces a myeloproliferative disorder with clinical and pathologic features of JMML62 (K.M. Shannon, personal communication, December 1996). Taken together these human and murine data point to deregulated signaling through the RAS pathway as the central event in the abnormal growth of JMML progenitor cells. Activation of the JAK-2 tyrosine kinase65 or overexpression of the Shc proteins57 might represent an additional mechanism(s) to be involved in GM-CSF hypersensitivity.
Schematic diagram of GM-CSF signal transduction from cell surface to nucleus. GM-CSF binds to the α and β subunits of its cell surface receptor. Two distinct signaling pathways are triggered by GM-CSF: the Ras signaling pathway and the one that involves activation of the Jak2 tyrosine kinase. The first one includes Shc phosphorylation, increases in GTP-bound Ras and activation of mitogen activated (MAP) kinases. The presence of RAS points mutations or the inactivation of neurofibromin (the protein encoded by the neurofibromatosis type 1 gene, NF1 ), constitutively activate the Ras pathway by increasing intracellular levels of Ras-GTP. Modified from Emanuel et al.23
Schematic diagram of GM-CSF signal transduction from cell surface to nucleus. GM-CSF binds to the α and β subunits of its cell surface receptor. Two distinct signaling pathways are triggered by GM-CSF: the Ras signaling pathway and the one that involves activation of the Jak2 tyrosine kinase. The first one includes Shc phosphorylation, increases in GTP-bound Ras and activation of mitogen activated (MAP) kinases. The presence of RAS points mutations or the inactivation of neurofibromin (the protein encoded by the neurofibromatosis type 1 gene, NF1 ), constitutively activate the Ras pathway by increasing intracellular levels of Ras-GTP. Modified from Emanuel et al.23
Several other cytokines secreted by malignant JMML cells have been implicated in autocrine or paracrine pathways of growth stimulation in JMML54 or in suppression of normal hematopoiesis.53 Bagby et al54 reported that monocytes from these patients secrete high levels of IL-1, which in turn stimulate the release of another growth factor(s) by normal accessory cells. In recent studies, an IL-1 receptor antagonist was reported to inhibit spontaneous JMML proliferation in vitro.66 Tumor necrosis factor (TNF ) α has been suggested to inhibit normal hematopoietic progenitors,53 resulting in anemia and thrombocytopenia, both clinical hallmarks of JMML.
The involvement of the erythroid lineage is a common feature of JMML, as indicated by the presence of mature but morphologically aberrant erythroid cells, the conversion to a fetal-type erythropoiesis, and the abnormal in vitro growth properties of peripheral erythroid progenitors.52,67 Cytogenetic analysis of burst-forming unit-erythroid (BFU-E) colonies from patients with a chromosome marker suggested that JMML may arise from early progenitors capable to differentiate into monocytic-macrophage–erythroid cells.38,39 The involvement of the erythroid lineage in this clonal disorder has been also confirmed by the observation of increased expression of GATA-1, erythropoietin receptor, and α and γ globin genes in JMML.68 More recently, loss of the normal NF1 allele in erythroblasts harvested from the BFU-E colonies of JMML patients has added support to this hypothesis.69
Several lines of evidence indicate the clonal nature of JMML. First, clonality was shown by the presence of a cytogenetic marker in the bone marrow of rare patients with JMML,38 and more recently by a clonal pattern of X chromosome inactivation in girls with this disease.56 Loss of the normal NF1 allele in CD34+ cells in some JMML patients represents a third independent line of evidence.69 Cell separation studies have also traced the origin of JMML stem cells to the level of the most primitive myeloid progenitor.56,69 Highly purified CD34+ CD38− early progenitor cells (as well as the reticulocytes, platelets, monocytes, and granulocytes) from a JMML patient have been shown to be clonally derived when examined by HUMARA (human androgen receptor assay).56 Loss of the normal NF1 allele in unfractionated bone marrow samples and in CD34+ cells lends further support to this view. The chronic and rapidly progressive (blast crisis) phase of JMML15 is likely to have the same clonal origin. In one report, a child with JMML and cytogenetic evidence of monosomy 7 developed pre-B acute lymphoblastic leukemia49; the karyotypic analysis of the lymphoblasts in this case confirmed the presence of monosomy 7, as typically seen in pure stem cell disorders such as the Ph-chromosome–positive CML, in which involvement of both lymphocytes and myeloid cells is well recognized.70 Whether JMML represents a true stem cell disorder or the consequence of malignant transformation occurring in hematopoietic cells restricted to the myeloid differentiation remains controversial. Miles et al69 recently reported the retention of the normal NF1 allele in cell lines derived from pre-B lymphocytes of a JMML patient carrying an NF1 gene mutation. However, Nf1 −/− cells can reconstitute both myeloid and lymphoid cells in mice, but abnormal proliferation is observed only in the myeloid compartment63 (K.M. Shannon, personal communication, December 1996). The recent successful engraftment of the human JMML stem cells in primary and secondary SCID mice should provide a model with which to further delineate the stem-cell properties of JMML.71
The relationship between JMML and childhood monosomy 7 syndrome is uncertain. Both disorders share several clinical features including a young age at diagnosis, a preponderance of male patients, prominent hepatosplenomegaly, myelomonocytic proliferation, increased frequency of NF1, and a poor prognosis when treatment consists of chemotherapy without marrow rescue.16,72-74 However, JMML patients without demonstrable monosomy 7 tend to have higher HbF levels,19 more pronounced lymphadenopathy, and more severe skin rashes than do patients with monosomy 7, who show a tendency to develop leukopenia and bacterial infections.16,72-75 The majority of patients with JMML do not have monosomy 7 at diagnosis, whereas others acquire the cytogenetic abnormality only at the time of the disease progression.28,41 In a study that failed to detect the loss of heterozygosity (LOH) of chromosome 7 in patients with JMML, Butcher et al76 speculated that JMML and monosomy 7 are distinct disorders that share some, but not all, of the multiple steps commonly seen in malignant transformation. This idea has gained further support from a recent study in which molecular analysis failed to show microscopic deletions of 7q in 22 cases of JMML.77
TREATMENT
The treatment of JMML continues to generate controversy. Early therapeutic attempts by Lilleymann, using sequential subcutaneous cytarabine and oral mercaptopurine, provided some improvement in the condition of patients, but not in survival time.13 Castro-Malaspina et al15 reported the failure of various treatment modalities, including chemotherapy with 6-mercaptopurine (with or without prednisone) in 27 patients and more intensive chemotherapy in four patients. Regardless of the type of regimen, chemotherapy never resulted in a complete remission (CR) in this large series. These investigators concluded that neither splenectomy nor radiotherapy nor moderate or intensive chemotherapy was of value in the treatment of JMML. Thus, during a period when vastly improved results were being reported for most childhood leukemias, the prognosis for JMML remained poor.
Experience over the past decade suggests that a subset of patients with JMML will become long-term survivors when treated exclusively with chemotherapy. Chan et al32 treated four children with an intensive AML-type regimen, three of whom relapsed after 11 to 21 months with one remaining in CR at 27 months follow-up. The outcome was superior to that in five children, who received less intensive therapy, all of whom died within 1 to 29 months. Despite obtaining hematological remission, ie, reversal of the blood counts to normal within 4 to 8 weeks of initiating therapy, with intensive chemotherapy, usually directed to AML, Festa et al33 were unable to eradicate extramedullary disease in their six patients, who all showed persistent hepatosplenomegaly and thus failure to achieve complete remission; all of the patients experienced hematologic relapse and died of disease at a median of 8 months. In his recent report on the use of intensive chemotherapy for childhood MDS, Hasle et al78 described 20 patients with this syndrome, including 8 with CMML who were treated with AML-type therapy; only 2 entered remission.
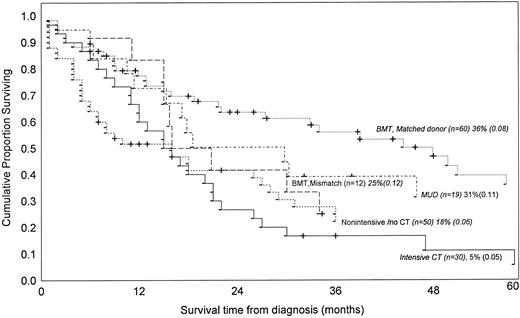
In our literature review we identified 171 children treated for JMML, with or without bone marrow rescue, for whom there was adequate information to correlate the duration of survival with the type of therapy. Fourteen of the 50 nonintensively treated patients (28%) were still alive after 1 to 307 months of follow-up (median 9 months), compared with only 3 of 30 (10%) who received intensive chemotherapy (surviving at 32, 36, and 120 months). Comparison of estimated survival probabilities suggests that intensive chemotherapy is not superior to nonintensive chemotherapy or even to no chemotherapy (Fig 3). These findings should be interpreted with caution because again we can only refer to published data, which were taken from sources without uniform entry criteria, did not consider proposed risk factors (Table 2) and do not represent randomized clinical trials.
Kaplan-Meier survival estimates of 171 children with JMML according to treatment applied as reviewed from the literature. The curves were generated by pooling the data from the following references, by including only cases for which both informations (ie, treatment applied and duration of survival) were available.13,15,19,20,27-29,31-35,37,40,43,47,79,82,83,85 97-103
Kaplan-Meier survival estimates of 171 children with JMML according to treatment applied as reviewed from the literature. The curves were generated by pooling the data from the following references, by including only cases for which both informations (ie, treatment applied and duration of survival) were available.13,15,19,20,27-29,31-35,37,40,43,47,79,82,83,85 97-103
The poor results of chemotherapy for JMML, with or without other modalities, have led to the evaluation of alternative agents, including cytokines and biological response modifiers. Castleberry et al79 recently described the efficacy of isotretinoin in producing durable clinical and laboratory responses in 10 children with this disease, two of whom had complete remissions, three partial remissions, and one a minimal response; the remaining four patients had progressive disease. Of the seven patients who did not undergo BMT, one was in complete remission 83 months after diagnosis, and two others had stable disease at 36 and 37 months while still receiving isotretinoin. Although clearly warranting further study, these data should be evaluated cautiously. First, a clinical response, defined by the authors as a reduction of leukocytosis, does not include the correction of cytopenia. Moreover, it is well known that some patients with JMML can have long periods of stable disease (up to 10 years), even when untreated. Additionally, the very young age of 10 patients who received isotretinoin (median, 10 months), probably had a favorable influence on outcome (Table 2).
The apparently increased sensitivity of the JMML cells to interferon-α,14 together with successful use of interferon for CML,80 prompted some investigators to administer this cytokine to patients with the juvenile form of the disease.15,29 The results are difficult to evaluate because of the limited sample size, the heterogeneity of the presenting clinical features and the complex history of previous treatment. Nonetheless, in one series of 10 previously untreated patients, high dose interferon therapy (30 millions units/m2) appeared excessively toxic with no evidence of improved disease control.81
Currently, bone marrow transplantation (BMT) is the only therapy that clearly improves outcome in the clinical management of JMML. Of 91 patients treated with this modality in 16 different reports, 38 (41%) were still alive, including 30 of the 60 patients who received grafts from HLA-matched or one antigen mismatched familial donor, 2 of 12 with mismatched donors, and 6 of 19 with matched unrelated donors. The estimated survival for patients treated with grafts from HLA-matched familial donors appears significantly better than results achieved with any other treatment modalities (Fig 3). Attempts to decrease the leukemic blast cell infiltrate or to ameliorate the degree of cytopenia, such as aggressive cytoreduction or splenectomy, may be helpful in some cases as a means to reduce the risk of peritransplant complications, but there is no evidence that such procedures may actually improve the survival rate or even reduce the risk of post-transplant leukemic relapse.83 The largest study of allogeneic BMT in JMML reported to date83,84 indicates that a preparative regimen consisting of busulfan and other cytotoxic drugs offers a higher likelihood of event-free-survival (due to lower rate of leukemic relapse) in children with an HLA-compatible relative donor than does any other modality including total-body-irradiation.85 As a result, this regimen is currently being used in the EWOG-BMT cooperative study.83
For patients lacking access to BMT, novel therapeutic approaches should be considered. For instance, the significant pathogenic role of IL-1 in JMML and the inhibitory effect of its neutralization on spontaneous cell proliferation in vitro66 provide a compelling rationale for experimental treatment with an IL-1 receptor antagonist. Additionally, evidence of a significant in vitro inhibitory effect of IL-4 on the JMML growth pattern and GM-CSF, TNF, and IL-1 secretion86 would appear to support therapeutic strategies based on this cytokine as well. Recently, IL-10 was demonstrated to inhibit the autonomous in vitro growth of marrow and peripheral blood mononuclear cells from JMML patients, most likely through suppression of endogenous GM-CSF release.87 This observation suggests therapeutic evaluation of IL-10 in JMML patients.
The potent effects of the human GM-CSF analogue E21R, which completely blocks effects of the growth factor on cell proliferation and induces apoptosis, might also offer a novel therapeutic approach.22 It may also be possible to use mutant Ras peptides as targets for specific immunotherapy.88 Finally, the observation that oncogenic Ras proteins are unable to transform tissue culture cells unless they are farnesylated suggests that FPTase inhibitors may be useful in the treatment of JMML or other neoplastic disorders in which RAS contributes to dysregulated cell growth.89,90 This concept is now under investigation in model systems.91-94
CONCLUSIONS
The diagnosis of JMML is based on evidence of splenomegaly, leukocytosis in the range of 20 to 30 cells × 109/L, monocytosis greater than 1.0 × 109/L, circulating myeloid precursors, and bone marrow hypercellularity with less than 20% myeloid blasts. Evidence of spontaneous growth of myelomonocytic cells in vitro, together with the verification of the absence of the t(9; 22) or the BCR/ABL fusion protein, should be obtained in all cases. High levels of HbF (usually >10%) are readily documented and strongly support a suspected diagnosis of JMML. Although JMML and monosomy 7 are probably two distinct entities, the presence of this cytogenetic abnormality should not be considered a diagnostic contraindication of JMML; patients with well-documented evidence of JMML and age greater than 1 year at disease onset should be considered at higher risk for rapidly progressive disease and thus possible candidates for early BMT from an HLA-identical sibling or an unrelated donor. Younger patients may benefit from nonintensive treatment, such as 6-mercaptopurine, 6-thioguanine, or isotretinoin. In general, patients should not be subjected to intensive chemotherapy or BMT with poorly matched donors unless their clinical course during the first few weeks or months is unusually aggressive or strongly suggestive of a blastic crisis. Experimental agents are justifiable in children with unfavorable risk features who lack an acceptable marrow donor. Meanwhile, it will be important to clarify the origin of fetal erythropoiesis in patients with JMML, the relationship between monosomy 796 and presenting clinical features, and the mechanisms that allow the disease to progress to blastic crisis.
ACKNOWLEDGMENT
The authors are indebted to Catherine Klersy, MD (Biometric Unit, Scientific Direction, IRCCS Policlinico S. Matteo, Pavia, Italy) for statistical analysis; Franco Locatelli, MD, Kevin Shannon, MD, Robert Castleberry, MD, and Charlotte Niemeyer, MD, for their critical comments and for sharing unpublished data; and John Gilbert for editorial review.
Supported in part by Grants No. PO1-CA-20180 and P30 CA-21765 from the National Cancer Institute (Bethesda, MD) (C.H.P.); by the American Lebanese Syrian Associated Charities (Memphis, TN) (C.H.P.); by Grant No. 390RCR91/01 from the IRCCS Policlinico S. Matteo, Pavia, Italy (M.A.); by Associazione Italiana per la ricerca sul Cancro (AIRC) (A.B.); by Consiglio Nazionale delle Ricerche (PF ACRO) No. 92.02140.PF.39 (A.B.); and by Fondazione Tettamanti (A.B.).
Address reprint requests to Maurizio Aricò, MD, Department of Pediatrics, University of Pavia, IRCCS Policlinico S. Matteo, 27100 Pavia, Italy.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal