Abstract
Understanding the repopulating characteristics of human hematopoietic stem/progenitor cell fractions is crucial for predicting their performance after transplant into high-risk patients following high-dose therapy. We report that human umbilical cord blood cells, 78% to 100% of which express the hematopoietic progenitor cell surface marker CD34, can consistently engraft, develop, and proliferate in the hematopoietic tissues of sublethally irradiated NOD/LtSz-scid/scid mice. Engraftment and development of CD34+ cells is not dependent on human growth factor support. CD34+ cells home to the mouse bone marrow (BM) that becomes the primary site of human hematopoietic development containing myeloid, lymphoid, erythroid, and CD34+ progenitor populations. Myeloid, and in particular lymphoid cells possessing more mature cell surface markers, comprise the human component of mouse spleen and peripheral blood, indicating that development proceeds from primary hematopoietic sites to the periphery. Repopulation of secondary recipients with human cells by BM from primary recipients demonstrates the maintenance of substantial proliferation capacity of the input precursor population. These data suggest that the cells capable of initiating human cell engraftment (SCID-repopulating cells) are contained in the CD34+ cell fraction, and that this mouse model will be useful for assaying the developmental potential of other rare human hematopoietic cell fractions in vivo.
MAMMALIAN HEMATOPOIESIS is a developmental progression that proceeds from a small pool of unique progenitor cells with long-term, multilineage potential to larger populations with more limited potential, to the mature blood cells found in the circulation.1 Characterization and quantification of these progenitor pools is fundamental to our understanding of this developmental sequence and is of great importance for human hematopoietic cell transplantation biology. Much of our understanding of the development and regulation of the mammalian hematopoietic system, including stem and progenitor cell potential and lineage relationships, has been derived from work in the mouse.1 In particular, the transplantation assays available in the mouse model have been instrumental in defining and characterizing the most primitive elements of the hematopoietic system; namely the long-term repopulating stem cell endowed with the potential to provide long-term, multilineage hematopoiesis following transplantation into appropriate recipients.2-4
As comparable experimental transplantation assays do not exist for human hematopoietic precursors, little is known about human long-term repopulating stem cells. The large majority of studies reported to date have attempted to extrapolate in vivo performance of a population from results of in vitro studies. Assays such as the colony forming unit-blast (CFU-blast),5 the high proliferative potential colony forming cell (HPP-CFC),6 and the long-term culture-initiating cells (LTC-IC)7 demonstrate the ability of a cell fraction to form colonies of erythroid, granulocyte/monocyte, and macrophage precursors over an extended time period in a defined, in vitro environment. However, a correlation has not been reported between these in vitro assay results and the reconstitution potential of the same cell fraction in vivo in a human hematopoietic environment. A further concern with these in vitro assays is that they are unable to predict the homing potential of particular populations.
Gaining a better understanding of the human long-term repopulating cell is essential as hematopoietic cell-supported, high-dose therapy is being employed with increasing frequency for a broad spectrum of high-risk malignancies and hematologic diseases. As a means of improving this therapy, potential new sources of hematopoietic stem cells such as umbilical cord blood (CB) and mobilized peripheral blood (PB) progenitor cells are currently under investigation. Additionally, the possibility of expanding primitive stem cells ex vivo is potentially a clinically useful strategy that is actively being pursued in many laboratories.8 9 A major concern with a number of these approaches is that the quality and number of stem cells in these populations are largely unknown. Consequently an experimental transplantation assay that could measure the repopulating potential of various human precursor fractions would be invaluable in furthering our understanding of these primitive hematopoietic progenitors.
Over the past decade, several groups have transplanted hematopoietic precursors into different mouse mutants in an attempt to develop a reproducible transplantation assay. In the SCID/hu model developed by McCune et al,10 a human hematopoietic microenvironment is created by implanting fragments of human fetal thymus and liver10 or bone11 into severe combined immunodeficiency disorder (C.B-17-scid/scid; SCID) mice. Hematopoietic precursor populations subsequently injected into these transplanted fetal tissues have been shown to proliferate and differentiate.11-13 This has been a particularly useful model for examining lymphocyte development in vivo.10,12 However few of the experimental cells in these models seed the mouse bone marrow (BM) or the peripheral hematopoietic tissues; instead they are generally restricted to the fetal explants. Models where human hematopoietic cells are transplanted intravenously into sublethally irradiated mice may more closely recapitulate the ability of particular human progenitors to home to primary hematopoietic sites as well as reflect their in vivo developmental potential. Such systems have been developed in beige/nude/x-linked immunodeficiency (BNX)14-17 and SCID18-22 mice. Unfractionated adult BM,14,17,19,20 PB,17,20 and CB,21 as well as fetal BM,17,22 have engrafted and been maintained for varying lengths of time in these animals. Maintenance of human cells in the mouse BM and periphery suggests human hematopoietic development can occur in the mice. Furthermore, Nolta et al15,16 have shown that when BM- or PB-derived CD34+ progenitor cells were cotransplanted with human interleukin-3 (IL-3)–expressing stromal cells into BNX mice, they could be maintained and generate cells of both the myeloid and lymphoid lineages. In most of these studies, high frequencies of engraftment or hematopoietic development have depended on treatment of mice with human cytokines or cotransplant with stromal cells engineered to produce human cytokines. In studies where growth factors were not used,17,21,22 high numbers of unfractionated input cells were required and/or engraftment was infrequent.17
Recently, the scid mutation has been backcrossed onto the nonobese diabetic (NOD/Lt) mouse background. In addition to lacking T- and B-cell function, the resulting NOD/LtSz-scid/scid (NOD/SCID) mice exhibit low natural killer cell activity, are defective in macrophage function, and lack hemolytic complement.23 NOD/SCID mice have shown improved engraftment over the SCID model when transplanted with unfractionated human spleen cells,24 BM leukocytes,25 PB mononuclear cells,26 and unfractionated CB cells.27 28
The improved ability to transplant human hematopoietic cells into NOD/SCID mice provides the foundation to develop a repopulation assay for primitive human cells. Although previous work has suggested that the cell capable of initiating the engraftment of human cells (termed the SCID-repopulating cell; SRC) is primitive,29 little work has been done to characterize the SRC. As such, this report is an initial characterization of hematopoietic development in NOD/SCID mice from the human CD34+ progenitor cell fraction of CB. Evidence is presented that CD34+ CB cells can consistently home to the BM and engraft in NOD/SCID mice. Furthermore, engrafted CD34+ populations can develop and give rise to myeloid and lymphoid cell lineages without the influence of exogenously supplied human growth factors. In addition, cells from the CD34 fraction with short-term myeloid progenitor potential are detected throughout the course of engraftment, while SRCs capable of repopulating secondary recipients are maintained for extended periods of time.
MATERIALS AND METHODS
Mice.Experimental NOD/SCID mice were obtained from a breeding colony established at the Biological Resource Center at the National Jewish Center (NJC; Denver, CO) from breeding pairs kindly provided by Leonard D. Shultz. The breeding colony is housed in a restricted barrier facility and the experimental animals were maintained in microisolator cages on laminar flow racks in a clean experimental room. Mice were maintained on an irradiated, sterile diet of Picolab Mouse Diet 20 (PMI Feeds, Inc, St Louis, MO) and given autoclaved, acidified water.
NOD/SCID mouse manipulations.All animal experiments were approved by the Animal Care Committees of the NJC and the University of Colorado Health Sciences Center. Immediately before cell transplantation 8- to 10-week-old NOD/SCID mice were injected intraperitoneally (IP) with 250 μL of phosphate-buffered saline (PBS) that contained 50 μL of reconstituted anti-asialo GM1 (Wako Chemicals, Inc, Richmond, VA). Identical treatments were performed on days 5 and 11 postinfusion of experimental cells. Mice were sublethally irradiated with 350 to 400 cGy from a 137Cs source immediately before intravenous tail-vein injection of 250 to 300 μL of Iscove's modified Dulbecco's medium (IMDM; GIBCO, Grand Island, NY) with 10% fetal bovine serum (FBS; Sigma Chemical Co, St Louis, MO) that contained an appropriate number of CB cells from the cell fraction being tested. In cases where secondary transplants were performed, BM cells were prepared from primary recipients as described below, pelleted 4 minutes at 720g and resuspended at 1.6 to 2.4 × 106/mL in IMDM/10% FBS. Secondary recipients each received 4 to 6 × 106 cells of the mouse/human cell suspension. For the duration of selected experiments, mice were injected IP three times per week with 250 μL PBS/5% FBS containing 10 μg each of the human growth factors IL-3, granulocyte-macrophage colony-stimulating factor (GM-CSF), and stem cell factor (SCF) (Amgen, Inc, Thousand Oaks, CA). After infusion of cells, mice were maintained in a HEPA-filtered isolator (Isotec, Bichester, UK) within an ultraclean barrier room for the duration of the experiment. The mice were killed in a CO2 chamber at predetermined time points. Blood was collected into heparinized tubes from pools formed in the chest cavity after the dorsal aorta was severed. Femurs and tibia were collected and aspirated with PBS/5% FBS to liberate BM cells. Cell suspensions were then filtered through sterile 70 μm Nitex to get rid of clumps and debris. The spleen and thymus (if present) from each mouse was harvested and strained through sterile 70 μm Nitex into PBS/5% FBS to collect cells.
CB CD34+ cell enrichment.Blood was aseptically aspirated from placenta and umbilical CB veins immediately following normal obstetrical deliveries at University Hospital (Denver, CO) and placed into blood bags containing an anticoagulant (citrate, phosphate, dextrose, adenine-1). Samples were processed within 8 hours of collection. Mononuclear cell (MNC) fractions were prepared by diluting blood 1:1.5 in PBS containing 300 U/mL DNase I (Sigma) and 1 mmol/L MgCl2, layering onto Ficoll (Lymphocyte Separation Medium, Organon-Technika, Durham, NC) and centrifuging for 30 minutes at 500g. The cells were washed in a buffer (PBS, 1 mmol/L MgCl2, 100 U/mL DNase I, and 1% human serum albumin), resuspended in the buffer and counted. CD34+ cell fractions were prepared from ficoll-separated MNCs using the buffer described above and the CD34 Progenitor Cell Isolation Kit (Miltenyi Biotec, Inc, Sunnyvale, CA) as per the manufacturer's directions. Cells were fractionated on a MACS column Type RS using a VarioMACS cell separator (Miltenyi Biotec, Inc). The eluted CD34 fraction was passed over a second RS column to obtain greater enrichment of CD34+ cells.
Flow cytometry.At least 5 × 104 nucleated cells were treated for lysis of erythrocytes with 1 mL hemolytic buffer (155 mmol/L NH4Cl, 12 mmol/L NaHCO3, 0.1 mmol/L EDTA) at room temperature for 2 minutes followed by washing with PBS/0.1% bovine serum albumin (BSA) and centrifugation at 500g for 5 minutes. To prevent nonspecific antibody binding, cells were resuspended for 20 minutes at 4°C in an antibody against Fc receptor (24.G230). Cells were washed and pelleted before being resuspended in 5 μg/1 × 106 cells of the appropriate antibody and incubated for 20 minutes at 4°C. Cells were then washed in PBS/0.1% BSA and resuspended in 0.5 mL of the same buffer. Two- or three-color flow cytometry was performed with a Coulter EPICS XL flow cytometer (Coulter, Hialeah, FL) using the software provided. Cells from a control mouse were stained with the same antibodies as a negative control. Appropriate isotype controls were also run for each fraction analyzed. Anti-human (hu) CD45-fluorescein isothiocyanate (FITC) and antimouse (mu) CD45-phycoerythrin (PE) were used to identify the ratio of human:mouse leukocytes. Specific subsets of human cells were quantified by gating on hu CD45-PerCP+ cells and then assessing staining with anti-hu: CD34-PE (multilineage progenitors), CD33-PE (myeloid progenitors) and CD13-FITC (myeloid), CD14-PE (monocytes) and CD42a-FITC (megakaryocytes), CD19-PE (B cells) and CD2-FITC (T cells), CD19-PE and IgM-FITC (developing B cells). Developing T cells were quantified by gating on hu CD3-PerCP+ cells and then assessing staining of anti-hu: CD4-FITC and CD8-PE. Nucleated human erythroid progenitors were assessed by quantifying hu CD71-FITC+/CD45-PerCP− cells in the hu glycophorin A-PE+ gate. A four parameter flow methodology was used for quantification of all CD34+ populations.31 All antibodies were from Becton Dickinson (San Jose, CA), except for anti-CD13 and anti-glycophorin A (Immunotech, Westbrook, ME), anti-mu CD45 (Pharmingen, San Diego, CA) and anti-hu IgM (Caltag, So. San Francisco, CA).
Cell sorting.Staining of CD34-selected fractions was performed as described above using anti-hu: CD34-PE, CD19-FITC, and either CD2-FITC or CD3-FITC. Cells were sorted aseptically into CD34+ CD19−/2−(3−) and CD34+ CD19+/2+(3+) fractions with a Coulter EPICS 752 flow cytometer using a Cicero acquisition system and Cyclops software (Cytomation, Ft Collins, CO).
CFU assay.Input cells, as well as BM cells from experimental mice at the time of death, were placed into a CFU assay. The number of mouse BM cells plated ranged from 1.47 to 3.57 × 105/plate, depending on the human cell content. One-milliliter aliquots of a mixture containing (vol/vol): 44% methylcellulose (Terry Fox Laboratories, Vancouver, BC), 30% FBS, 10% BSA, 1% 0.1 mmol/L methylprednisolone, 1% 11 mmol/L β-mercaptoethanol, and 14% IMDM containing the appropriate number of cells, plus 1 U/mL human erythropoietin, 10 ng/mL each of human: IL-3, GM-CSF, and SCF (Amgen, Inc) were plated in quadruplicate into 30 cm2 dishes (Falcon 1008; Becton Dickinson, Lincoln Park, NJ) as previously described.32 After 14 to 15 days at 37°C, the plates were scored for colony forming cells that included granulocyte/monocyte (CFU-GM), burst-forming units-erythroid (BFU-E), and granulocyte/erythroid/megakaryocyte/macrophage (CFU-GEMM).
Polymerase chain reaction (PCR) for hu Cart-1.Individual colonies for analysis were plucked from CFU plates and placed into tubes containing PBS. Cells were washed once in PBS and resuspended in 25 μL 0.1× PBS in PCR tubes. The tubes were heated to 95°C for 8 minutes in a thermocycler. One μL proteinase K (20 mg/mL; Boehringer Mannheim, Indianapolis, IN) was added to each tube and the tubes were placed back in the thermocycler and held at 56°C, 30 minutes and then at 94°C, 8 minutes. Tubes were spun and supernatant used for PCR reactions. PCR reactions contained 1× PCR buffer (Perkin-Elmer, Branchburg, NJ), 1.5 mmol/L MgCl2, 0.1 mmol/L nucleotide triphosphates, 0.125 μL AmpliTaq polymerase (Perkin-Elmer) preincubated for 5 minutes at room temperature with 0.138 μg TaqStart antibody (Clontech, Palo Alto, CA), 1 μmol/L of each specific primer, 10 μL sample, and dH2O to make a final reaction volume of 25 μL. The “hot start” reaction was performed in a Perkin-Elmer 9600 and included a pre-melt for 2 minutes at 94°C followed by 30 cycles of 94°C, 30 seconds, 58°C, 1 minute, 72°C, 45 seconds, and a final primer extension at 72°C for 7 minutes. Specific primers included: 5′-aaggataccacaataagctgc-3′, and 5′-ggtttgtggagactggcac-3′. This primer set yields a 156-bp product from the 3′ untranslated region of the human Cart-1 gene and fails to amplify a product from mouse genomic DNA.33 Positive and negative controls for human specificity of the primers included using template extracted from either 1,000 human PB white cells or 1,000 mouse BM cells, respectively. As a control for the presence of sufficient DNA template, parallel PCR reactions were performed on each sample using primers specific for β-actin. This primer set consisted of: 5′-aaggccaaccgcgagaagat-3′ and 5′-tcggtgaggatcttcatgag-3′, that amplifies a 249-bp fragment from control genomic DNA.34 The reaction conditions were identical to those described above except TaqStart antibody was not used.
RESULTS
Phenotype of input cells.To obtain highly enriched CD34+ cell fractions for use as input cells, double immunoselection was performed on most fractions. In two experiments (5 and 8), the cells were immunomagnetically fractionated once and then purified further by fluorescence-activated cell sorting (FACS). Typically the yield following immunomagnetic selection from a single CB was in the range of 0.5 to 2.0 × 106 cells and thus limited the amount of input cell phenotyping that was feasible. To determine the phenotype of input cells, four CB samples each were fractionated by double immunomagnetic selection and the resulting populations (Control CB fractions in Table 1) were used to assess the frequency of cells bearing the surface markers subsequently measured in output cells from the experiments. Antibody combinations and flow gating used for analysis were identical to those used for experimental output cells. CD34+ cell purity ranged from 75% to 95% in control fractions (Table 1). Purities of CD34+ fractions used in transplant experiments fell within this range or were above it (Table 1). Table 1 shows that myeloid progenitor cells (CD33+) comprised a consistent, albeit low, percentage of CD34 fractions, while more mature myeloid cells (CD13+ and CD14+) were largely absent. Development from CD34+ cells into the myeloid lineages could thus be detected in mice using these fractions. Erythroid progenitor cells (glycophorin A+CD71+CD45−) were present as well and ranged from 0.15% to 3.8% of the fractions. The major contaminants in CD34 fractions were B cells (CD19+) and T cells (CD2+). To distinguish between clonal expansion of CD19+ or CD2+(3+) lymphoid cells and lymphoid development from CD34+ cells in mice, CD19+ and CD2+(3+) cells were eliminated from two input cell fractions (experiments 5 and 8; see Table 1) by FACS.
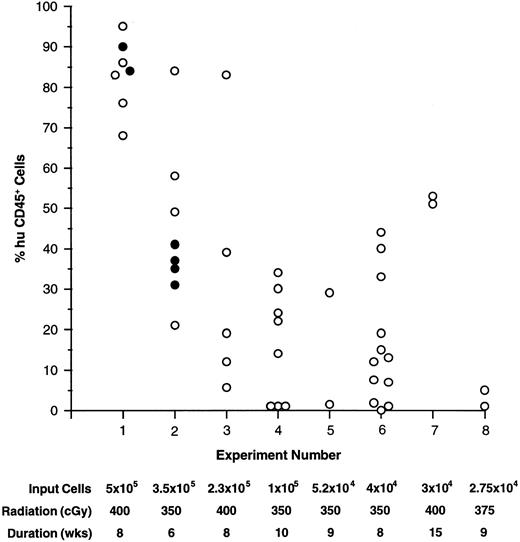
Engraftment into mice.All CD34+ cell-enriched fractions used to transplant NOD/SCID recipients to date have successfully engrafted the mice (Fig 1). Short-term hematopoietic development was analyzed after 6 to 10 weeks in most experiments, although engraftment persisting through 15 weeks was demonstrated in experiment 7 (Fig 1). In several experiments the extended engraftment potential of CD34 fractions was assessed by serial transplantation of BM from primary recipients (see below). Although the BM was the primary site of human cell engraftment and proliferation, human cells were also detected and analyzed in the spleen and PB of all recipients. The fraction of human cells in these organs ranged from 1% to 30% and was always lower than that detected in BM. In two mice (from experiments 1 and 3), the thymus was found to contain human cells exclusively. It should be stressed that repopulation of mice thymi with human cells was not a consistent finding, but rather, a rare event. Although a limiting dilution experiment was not performed, the level of engraftment generally fell off with decreasing numbers of input cells (Fig 1). However, as few as 2.75 × 104 CD34+ cells (experiment 8) were capable of yielding detectable engraftment levels.
Engraftment of human CD34+ cell-enriched fractions into NOD/SCID BM. The percent of huCD45+ cells found in NOD/SCID BM are plotted for individuals in experiments 1 through 8. The number of input cells, pretreatment radiation dose, and duration of the experiment are listed below each experiment number. In experiments 1 and 2, 10 μg each of human: IL-3, GM-CSF, and SCF was administered three times per week to one group of mice (•), while others were given no growth factors (○).
Engraftment of human CD34+ cell-enriched fractions into NOD/SCID BM. The percent of huCD45+ cells found in NOD/SCID BM are plotted for individuals in experiments 1 through 8. The number of input cells, pretreatment radiation dose, and duration of the experiment are listed below each experiment number. In experiments 1 and 2, 10 μg each of human: IL-3, GM-CSF, and SCF was administered three times per week to one group of mice (•), while others were given no growth factors (○).
Conditions were assessed that affected engraftment of CD34 fractions into recipients such as the pretreatment radiation dose and the need for administration of human growth factors. Initial experiments were performed by irradiating mice with 400 cGy. At this dose 65% (26 of 40; experiments 1, 3, and 7) of the animals died 1 to 2 weeks posttransplant. Surviving mice generally engrafted well, however acute deaths, most likely due to radiation-induced toxicity, were considered too extreme. In experiments where mice received 350 (experiments 2 and 4 through 6) or 375 (experiment 8) cGy, engraftment levels were slightly lower than in mice that had received higher doses, but the number of early deaths was dramatically reduced to 20% (eight of 40). For later experiments, 350 cGy was used routinely for pretreatment.
In previous studies, consistent engraftment and development of CD34+ cells has relied on the influence of human growth factors.15,16 Alternatively, unfractionated CB (comprised of cells known to produce growth factors) has been shown to engraft mice to comparable levels with and without growth factor supplements.21 Two experiments were performed (1 and 2) to assess the influence of human: IL-3, GM-CSF, and SCF supplied three times per week on engraftment and subsequent development of CD34 fractions. Figure 1 illustrates that engraftment levels in mice that received no growth factor treatments (open circles) were comparable to those in mice that received growth factors (closed circles). Additionally, significant differences were not detected in output human cell subpopulations between treated and untreated animals (Fig 2 and see below). Because of these findings, subsequent experiments were done without the use of growth factors.
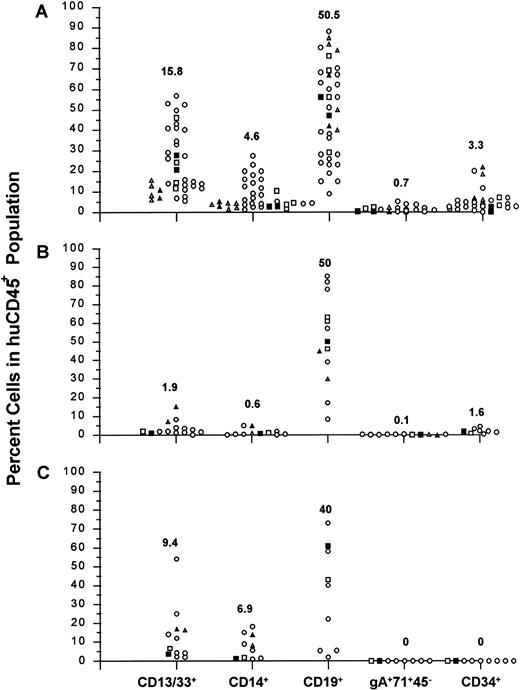
Summary of huCD45+ cell subpopulations found in mouse BM (A), spleen (B), and PB (C). The percent of the huCD45+ population that also expressed human myeloid cell (CD13/33+ and CD14+), B cell (CD19+), erythroid progenitor cell (gA+71+45−), and CD34+ cell surface markers is shown for all samples analyzed from experiments 1 through 8. Median values are shown above each subpopulation. Samples are identified from experiments 1 (▴, ▵) and 2 (▪, □) where mice received either 10 μg each of human: IL-3, SCF, and GM-CSF, three times per week (▴, ▪), or received no growth factor treatments (▵, □).
Summary of huCD45+ cell subpopulations found in mouse BM (A), spleen (B), and PB (C). The percent of the huCD45+ population that also expressed human myeloid cell (CD13/33+ and CD14+), B cell (CD19+), erythroid progenitor cell (gA+71+45−), and CD34+ cell surface markers is shown for all samples analyzed from experiments 1 through 8. Median values are shown above each subpopulation. Samples are identified from experiments 1 (▴, ▵) and 2 (▪, □) where mice received either 10 μg each of human: IL-3, SCF, and GM-CSF, three times per week (▴, ▪), or received no growth factor treatments (▵, □).
Hematopoietic development.BM, spleen, and PB were harvested from mice 6 to 15 weeks after infusion of CB CD34+ cells and the fraction of human cells present in each hematopoietic tissue was determined by costaining cells with antibodies specific for both mouse and human CD45, respectively. The cellularity of the tissues in all recipients was normal. At the levels of engraftment obtained (Fig 1), a significant expansion of human cells in the mice occurred over the course of the experiments. For instance, the BM (from two femurs and two tibia) of an individual mouse in experiment 4 contained 3.25 × 107 cells, of which 30% were human. Thus, 9.75 × 106 human cells in the femurs and tibia were derived from 1 × 105 input cells, representing an increase of at least 500-fold (the femurs and tibia represent only 20% of the BM cavity35 ). In general, increases of human cell numbers were observed in all mice, indicating that the mouse environment was conducive to proliferation of human hematopoietic cells.
Cells from the tissues were further analyzed by three-color flow cytometry for evidence of multilineage development from input CD34+ populations. Cells within the huCD45 gate were quantified for myeloid, lymphoid, and erythroid cell surface markers, as well as for the retention/expansion of a CD34+ population. Figure 3A shows representative analysis of BM, spleen, and PB, while Fig 2 summarizes the values of each huCD45+ subfraction for all samples analyzed in experiments 1 through 8. The myeloid component of BM ranged from 5% to 57% of the human population. CD13+ and CD13+/33+ cells made up the majority of these populations, while CD14+ cells comprised a smaller proportion of huCD45+ cells. The fraction of myeloid cells in the huCD45+ gate varied between experiments, as well as between individuals within experiments. B cells were the major human component of BM, in most cases, comprising 8.4% to 85% of the huCD45+ cell population. Erythroid progenitors were consistently found in low frequencies. BM from the majority of mice analyzed contained a substantial proportion of CD34+ cells. The proportion of CD34+ cells did not depend on the duration of the graft. Mice analyzed at 6 weeks had 1.5% to 6.3% CD34+ cells, while mice analyzed at 15 weeks retained 2.5% to 6.9% CD34+ cells in their BMs.
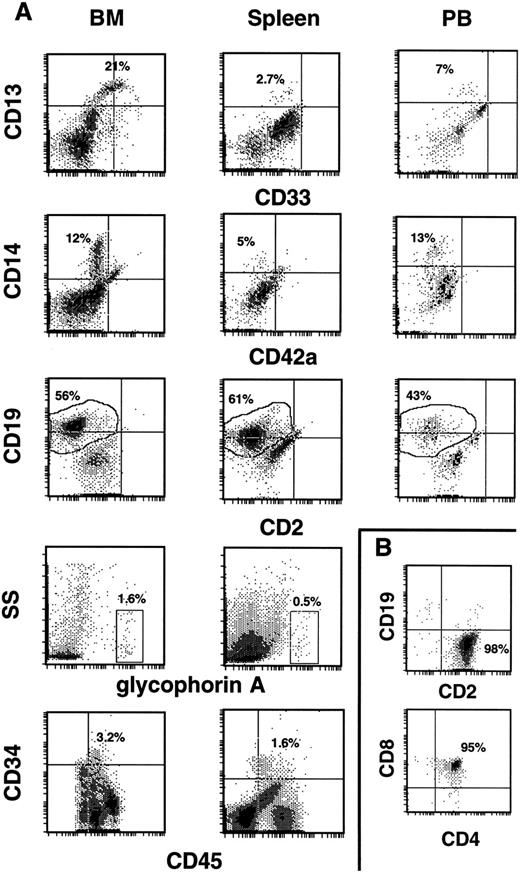
Development of human CD34+ cells in the hematopoietic tissues of NOD/SCID mice. (A) Typical profiles of human progenitor cell (CD34, CD33), myeloid cell (CD13, CD14), B-cell (CD19), and T-cell (CD2) surface markers on huCD45+ output cells from the BM, spleen, and PB of repopulated mice. Cells in the glycophorin A+ gate were 100% huCD71+ and 0% to 3% huCD45+. Quantification of the CD34+ population was done by using a four parameter gating method.31 (B) In the rare cases where huCD45+ cells populated the thymus (two mice), the vast majority (98%) were huCD2+ (upper histogram). When the thymic population was gated on huCD3+ cells (which comprised 72% of the total cells), 95% were hu CD4/CD8 double positive cells (lower histogram).
Development of human CD34+ cells in the hematopoietic tissues of NOD/SCID mice. (A) Typical profiles of human progenitor cell (CD34, CD33), myeloid cell (CD13, CD14), B-cell (CD19), and T-cell (CD2) surface markers on huCD45+ output cells from the BM, spleen, and PB of repopulated mice. Cells in the glycophorin A+ gate were 100% huCD71+ and 0% to 3% huCD45+. Quantification of the CD34+ population was done by using a four parameter gating method.31 (B) In the rare cases where huCD45+ cells populated the thymus (two mice), the vast majority (98%) were huCD2+ (upper histogram). When the thymic population was gated on huCD3+ cells (which comprised 72% of the total cells), 95% were hu CD4/CD8 double positive cells (lower histogram).
Figures 2 and 3A reveal that CD13+ and CD14+ myeloid cells were consistently found in the spleen and in the peripheral circulation of recipients, albeit generally at lower levels than that seen in BM. Much lower proportions of myeloid cells were found in the spleen than in PB. As in BM, a greater proportion of huCD45+ cells expressed CD13 than CD14, while the majority of cells expressed CD19 (Figs 2 and 3A). It should be noted that the myeloid compartment seen in these organs consisted of CD13+ and CD14+ cells and generally lacked the CD33+ and CD13+/33+ populations that were found in BM (compare histograms in Fig 3A). Erythroid progenitors were found in over half of the spleens analyzed and were generally low in frequency. Erythroid progenitors were not detected in PB. CD34+ progenitor cells were consistently found in spleen at lower frequencies than in BM. Again, no CD34+ cells were detected in PB samples (Figs 2 and 3A). Figure 2 also shows that there were no significant differences in subpopulation distributions found in BM, spleen, or PB samples between mice supplemented with human growth factors and those that were not (experiments 1 and 2).
A grossly visible thymus was usually absent from mice at the time of killing. In mice where the thymus was seen, it was collected and analyzed. Most contained mouse CD45+ cells exclusively that were probably a prelude to thymoma, which develops in these mice after 5 to 7 months of life.23 However, two mice (one each from experiments 1 and 3) had a thymus that contained 1 to 2 × 107 cells that were 91% to 96% huCD45+. Within this population, 98% of the cells were CD2+ (Fig 3B, top histogram). When cells in this population were stained with anti-hu: CD3, CD4, and CD8, it was found that the majority (95%) were CD3+/CD4+/CD8+ (Fig 3B, bottom histogram). Input cells consistently were contaminated with a CD2+ population (Table 1). However, analysis of two CD34+ input cell fractions showed the presence of no CD4/CD8 (double positive) cells, suggesting the human T cells found in these thymi represent de novo lymphopoiesis in the mouse thymic environment. The basis for the inconsistent nature of thymic colonization by human CD34+ cells remains to be determined.
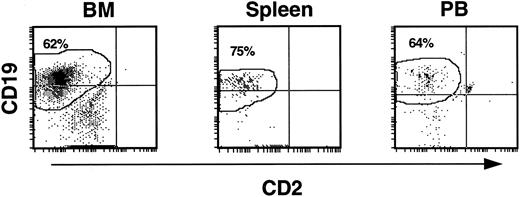
As lymphoid cells were a consistent contaminant of input cell CD34 fractions, the CD19+ cells observed in mouse hematopoietic tissues might have been due to expansion of cells already committed to the B-cell lineage. To determine if CD19+ cells were indeed generated from more immature precursors, CD19+ cells were removed from CD34 fractions by FACS before infusion into mice (experiments 5 and 8). Analysis of these experiments showed that myeloid, lymphoid, erythroid, and progenitor cell levels in BM, spleen, and PB fell within the range of values seen in the other experiments. CD13+/33+ cells made up 6% to 26%. CD14+ cells ranged from 2.6% to 8.4%, and CD34+ cells comprised 2% to 18.6% of the huCD45+ population. Importantly, CD19+ cells were the major population of human cells in the BM, spleen, and PB of all animals (Fig 4). In BM, CD19+ cells accounted for 28% to 62% of the human leukocytes. These cells were derived in the mice from SRCs within a CD34 fraction containing cells with no detectable expression of CD19, suggesting that cells in this system can differentiate down the lymphoid pathway from CD34+ cells devoid of an early B-cell surface marker.
CD19+ B-cell development from CD34+CD19− input cell populations in NOD/SCID recipients. Elimination of contaminating CD19+ cells by FACS from input CD34 cell fractions had no effect on the generation of B-cell precursors in the mice. The histograms show that, as in other experiments, output cells expressing CD19 predominated in the BM, spleen, and PB of repopulated mice.
CD19+ B-cell development from CD34+CD19− input cell populations in NOD/SCID recipients. Elimination of contaminating CD19+ cells by FACS from input CD34 cell fractions had no effect on the generation of B-cell precursors in the mice. The histograms show that, as in other experiments, output cells expressing CD19 predominated in the BM, spleen, and PB of repopulated mice.
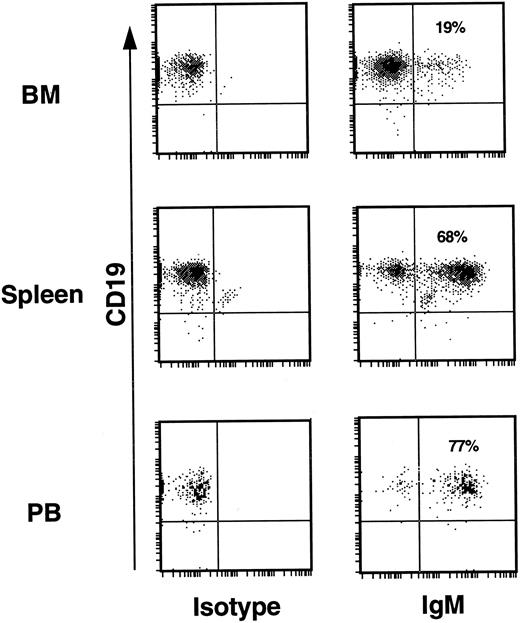
The huCD19+ populations of BM, spleen, and PB were analyzed for cell surface expression of hu IgM as evidence of further B-cell development. For this analysis, human CD45+ BM, spleen, and PB cells from repopulated mice were stained with both anti-hu CD19 and anti-hu IgM. Human CD45+ cells from the lymph gate of the light scatter profile are shown in Fig 5. Without exception, all mice analyzed showed similar patterns of expression in the three hematopoietic compartments. This included mice that had been transplanted with CD34+CD19− cells from experiments 5 and 8 where both more mature CD19+ cells, as well as CD34+CD19+ pro B cells were absent from the input cell fraction (Table 1). In the BM, the majority of CD19+ cells expressed no cell surface IgM; a finding that would be expected in a primary lymphopoietic site. In contrast to the BM, approximately two thirds of the CD19+ cells in the spleen expressed IgM, indicating a more mature B-cell population. In PB, 75% to 90% of the CD19+ population expressed IgM. These findings suggest that human B-cell maturation in mice is correlated with a progression of cells from the site of primary lymphopoiesis, the BM, to the peripheral circulation, similar to the situation in humans.
B-cell development. In the BM of mice from all experiments only 10% to 20% of huCD19+ cells expressed cell surface IgM. In the spleen 60% to 75% of CD19+ cells also expressed IgM. PB from all mice revealed that 75% to 90% of huCD19+ cells also expressed IgM. This was true for mice transplanted with input CD34+ cell fractions known to be contaminated with low levels of CD19+ cells (experiments 4 and 7, Table 1), as well as for mice transplanted with CD34+ input fractions devoid of any CD19+ cells (experiments 5 and 8, Table 1).
B-cell development. In the BM of mice from all experiments only 10% to 20% of huCD19+ cells expressed cell surface IgM. In the spleen 60% to 75% of CD19+ cells also expressed IgM. PB from all mice revealed that 75% to 90% of huCD19+ cells also expressed IgM. This was true for mice transplanted with input CD34+ cell fractions known to be contaminated with low levels of CD19+ cells (experiments 4 and 7, Table 1), as well as for mice transplanted with CD34+ input fractions devoid of any CD19+ cells (experiments 5 and 8, Table 1).
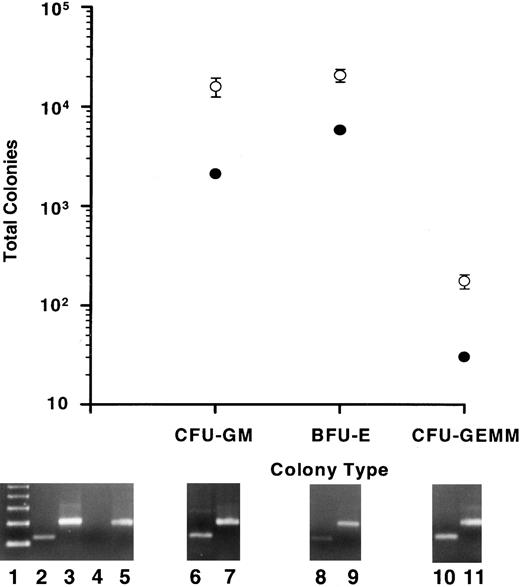
Colony-forming potential in vitro.As a further indication of human myelopoiesis, the human hematopoietic colony-forming potential was determined for output cells from the BMs of repopulated mice. The CFU assay was performed on output cells from mice in all experiments. BM from all mice exhibited the same levels of output cell colony-forming potential relative to that of input cells, including those mice that were treated with human growth factors. Data from experiment 4 are presented in Fig 6 as representative. A substantial number of CFUs, that included CFU-GM, BFU-E, and CFU-GEMM, were found in the BMs of all mice analyzed (Fig 6, open circles). Values for total CFUs were calculated by multiplying the number of CFUs counted by the fraction of total huCD45+ cells/huCD45+ cells plated for both input cells and output cells from each mouse. Figure 6 (closed circles) reveals that the total colonies derived from 1 × 105 input cells included 2.09 × 103 CFU-GM, 5.88 × 103 BFU-E, and 30 CFU-GEMM. Total output cell CFUs were calculated for BMs of five mice (range of huCD45+ cell populations, 14% to 34%) 10 weeks postinfusion of 1 × 105 input cells and included (mean ± standard error [SE]): 1.6 × 104 ± 3.4 × 103 CFU-GM, 2.08 × 104 ± 3.05 × 103 BFU-E, and 176 ± 29 CFU-GEMM (Fig 6, open circles). These are the minimum number of human CFUs derived from the mice; cells capable of producing myeloid colonies that were not taken into account included the BM fraction excluded from the femurs and tibia and the spleen cell population.
Human myeloid colony-producing potential in vitro of input cell fractions and of output cells from the BMs of repopulated mice. Total GM, erythroid (BFU-E), and GEMM colonies are plotted for 1 × 105 input cells from experiment 4 (•). BM cells from five mice that had each been infused with 1 × 105 input cells and allowed to engraft for 10 weeks were plated into the CFU assay. The means and SE of the total CFU in each category are plotted (○). All colonies analyzed by a human-specific PCR (at least 30 colonies/CFU type/mouse) were of human origin. Typical PCR signals are shown below specific colony types for human Cart-1 (lanes 6, 8, and 10) and for β-actin (lanes 7, 9, and 11). Controls consisted of PCRs for human Cart-1 (lanes 2 and 4) and β-actin (lanes 3 and 5) from human PB leukocytes (lanes 2 and 3) and normal mouse BM cells (lanes 4 and 5). Lane 1 shows a 123-bp ladder. The predicted amplified products for the hu Cart-1 PCR and for the β-actin PCR are 156 and 249 bp, respectively.
Human myeloid colony-producing potential in vitro of input cell fractions and of output cells from the BMs of repopulated mice. Total GM, erythroid (BFU-E), and GEMM colonies are plotted for 1 × 105 input cells from experiment 4 (•). BM cells from five mice that had each been infused with 1 × 105 input cells and allowed to engraft for 10 weeks were plated into the CFU assay. The means and SE of the total CFU in each category are plotted (○). All colonies analyzed by a human-specific PCR (at least 30 colonies/CFU type/mouse) were of human origin. Typical PCR signals are shown below specific colony types for human Cart-1 (lanes 6, 8, and 10) and for β-actin (lanes 7, 9, and 11). Controls consisted of PCRs for human Cart-1 (lanes 2 and 4) and β-actin (lanes 3 and 5) from human PB leukocytes (lanes 2 and 3) and normal mouse BM cells (lanes 4 and 5). Lane 1 shows a 123-bp ladder. The predicted amplified products for the hu Cart-1 PCR and for the β-actin PCR are 156 and 249 bp, respectively.
To confirm that the colonies grown in the CFU assay were of human origin, all GEMM colonies, as well as 30 GM and 30 BFU-E colonies (representing 3.5% to 10.6% and 5.4% to 8.4% of the total GM and BFU-E colonies, respectively, derived from each mouse) were plucked from CFU plates of each mouse and analyzed by a human-specific PCR (Fig 6). While DNA extracted from untransplanted, control mouse BM cells showed no amplified product, every colony analyzed from output cell CFU plates produced a product of the same size as one amplified from human white blood cells (≈150 bp; Fig 6). Using an exact nomagram for bimodal proportions and using 100% as the estimated proportion and analysis of at least 60 colonies/mouse, the confidence level is 95% that from 94% to 100% of the colonies from each mouse were human. These results strongly suggest that only human colonies were able to proliferate under the conditions used in this CFU assay.
Taken together, these data suggest that substantial human myelopoiesis and erythropoiesis occurs in mouse BM as demonstrated by the significant number of human colony-forming cells identified.
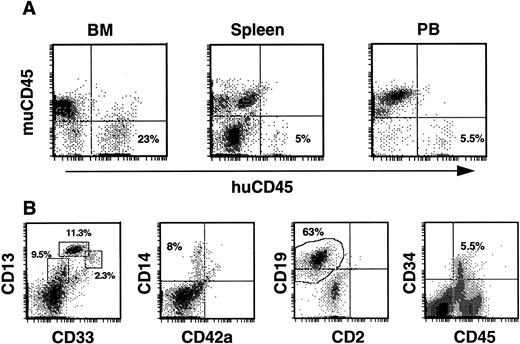
Secondary transplants.As phenotypic analysis of primary recipients indicated maintenance of a human CD34+ population, the next series of experiments was aimed at determining whether the human cell fraction contained SRC with the capacity for more durable, multilineage engraftment. In these experiments, we evaluated the ability of BM from previously engrafted mice to repopulate secondary recipients with human cells. BM of one mouse from experiment 1 (83% huCD45+ after 8 weeks) was transplanted into three recipients (calculated human CD34+ cells received/mouse was 6.8 × 105) and BM of a second mouse, from experiment 7 (51% huCD45+ after 15 weeks), was transplanted into two recipients (calculated human CD34+ cells received/mouse was 2.1 × 105). Two of the three mice from the first transplant died 7 and a half weeks posttransplant, however significant human engraftment was found in the BM and periphery of the remaining mouse 8 weeks posttransplant. Human CD45+ cells comprised 23% of BM cells, 5% of spleen cells, and 5.5% of PB cells in this mouse (Fig 7A). Within the huCD45+ gate of the BM the levels of developing progenitor, myeloid and lymphoid cells paralleled those seen in primary transplants. Specifically, 23% expressed CD13/33, 8% expressed CD14, 63% expressed CD19, and 5.5% expressed CD34 (Fig 7B). Thus, a CD34+ population was maintained at levels equal to those in primary recipients. In the spleen, the majority of huCD45+ cells expressed CD19 (80%), while 8% expressed CD13/33, 4% expressed CD14, and no CD34+ cells were detected (not shown). Human CD45+ cells in the PB consisted of CD13+ (13%), CD14+ (5.7%), and CD19+ (61%) populations (not shown). SRCs in the original CD34-enriched fraction, therefore, were capable of sequentially repopulating two mice with myeloid, lymphoid, and progenitor cells over a total period of 4 months.
Human engraftment of secondary recipients by BM from a primary recipient of human CD34+ cells. (A) Histograms of BM, spleen, and PB cells stained with antibodies against both mu and hu CD45. All secondary recipients from experiments 1 and 7 contained huCD45+ cells in these tissues. (B) Typical profiles of the myeloid, lymphoid, and progenitor cells comprising the huCD45+ cell fraction of mouse BM are illustrated with histograms from the BM of a secondary recipient. Mouse BM cells were triple-stained and cells within the huCD45+ gate were analyzed. Populations of myeloid progenitors (CD33+, 2.3%), early myeloid cells (CD13+/33+, 11.3%), more mature myeloid cells (CD13+, 9.5%), monocytes (CD14+, 8%), and B cells (CD19+, 63%) were observed, while a significant proportion (5.5%) of huCD45+ BM cells expressed CD34.
Human engraftment of secondary recipients by BM from a primary recipient of human CD34+ cells. (A) Histograms of BM, spleen, and PB cells stained with antibodies against both mu and hu CD45. All secondary recipients from experiments 1 and 7 contained huCD45+ cells in these tissues. (B) Typical profiles of the myeloid, lymphoid, and progenitor cells comprising the huCD45+ cell fraction of mouse BM are illustrated with histograms from the BM of a secondary recipient. Mouse BM cells were triple-stained and cells within the huCD45+ gate were analyzed. Populations of myeloid progenitors (CD33+, 2.3%), early myeloid cells (CD13+/33+, 11.3%), more mature myeloid cells (CD13+, 9.5%), monocytes (CD14+, 8%), and B cells (CD19+, 63%) were observed, while a significant proportion (5.5%) of huCD45+ BM cells expressed CD34.
Both mice transplanted with mouse BM from experiment 7 survived and were analyzed 9 weeks posttransplant. BMs from the mice contained 10% and 6% huCD45+ cells, respectively, while spleens and PBs from both mice contained 1% to 2% huCD45+ cells. Subpopulation analysis of huCD45+ cells showed results similar to those described above. To date, the original CD34-enriched fraction has been capable of engrafting and developing in mice for 4 months while maintaining cells able to repopulate secondary recipients for an additional 2 months, resulting in a total engraftment time of 6 months. Although only a limited number of secondary recipients have been analyzed, these data suggest that cells maintained in primary recipients did retain some repopulation capacity.
DISCUSSION
Evidence presented in this report establishes that CD34-enriched cells derived from human CB and infused intravenously consistently engraft NOD/SCID recipients. Over 90% of recipients that survived to the end of the experimental period showed varying degrees of human cell repopulation. As in human hematopoietic progenitor cell transplants, BM was the primary site of repopulation, but peripheral hematopoietic organs showed significant repopulation with human cells as well. This is the first report showing that the CD34+ progenitor cell fraction of human CB can engraft and proliferate throughout the hematopoietic tissues of NOD/SCID recipients.
For mice to survive in reasonable numbers following successful engraftment, the pretreatment radiation dose was crucial. At 400 cGy, 65% of recipients died within the first 2 weeks following radiation treatment. This is perhaps not surprising, as the SCID mutation is known to confer high radiation sensitivity to the animals.36 37 However, at a dose of 350 cGy, only ≈15% of the mice died acutely following irradiation. While at the higher radiation dose, surviving mice generally showed the highest levels of engraftment (5% to 95%), levels found at the lower radiation dose, where huCD45+ cells in the BM generally ranged from 10% to 60%, were adequate for the developmental analysis of human cells.
In other mutant mouse models, human growth factor supplements were required to obtain substantial proliferation of transplanted human BM or PB cells.14-16,18-20 In this system, engraftment and development of CD34+ CB cells in the absence of exogenous human growth factors was evident over the entire range of input cell doses used. In two of the experiments (1 and 2), where input CD34+ cell doses were 5 × 105 and 3.5 × 105, respectively, the level of engraftment of CB progenitors, as well as the output cell subpopulation distributions and colony-forming potential, were largely unaffected by the influence of human IL-3, GM-CSF, and SCF. Previous studies have shown that unfractionated CB can engraft without the addition of exogenous growth factors.21 However, this population contains cell types (eg, T cells) that could be producing growth factors in vivo. This is the first report in a mouse xenotransplant system where human hematopoietic development has been seen from CD34+ progenitor cells without growth factor supplementation. This is significant because high levels of human growth factors are not normally found systemically and could alter the engraftment potential of the input cell population. This data also suggests that cells contained in the CD34+ CB population possess an intrinsic ability for engraftment. Alternatively, or perhaps in addition, the NOD/SCID stroma may be a particularly favorable environment for supporting engraftment of these cells.38 The fact that growth factor supplements are not required for engraftment, development, and proliferation of CD34+ cells may be advantageous for future studies in determining the in vivo developmental potential of rare human progenitor cell populations without the bias that specific growth factors may introduce.
The human CD34+ cells infused into mice did not randomly distribute and proliferate throughout the mouse hematopoietic organs, but instead were partitioned in a manner consistent with normal hematopoiesis. Human CD34+ cells were found primarily in the mouse BM, as were the most immature populations of B cells (predominantly CD19+IgM−) and myeloid cells (CD13+/33+). In addition, erythroid precursors were found primarily in the BM. In mouse PB, more mature populations of myeloid (CD13+) and B cells were found (predominantly CD19+IgM+), while no populations of CD34+ cells or of erythroid progenitors were detected. This finding suggests not only that mouse BM was the primary site of human hematopoiesis, but that cells capable of engrafting mice in the original input CD34 fraction homed to the mouse marrow. Human CD34+ cells, at least to some extent, must recognize and adhere to the appropriate environment in the mouse BM, suggesting that this homing mechanism may be conserved between these species. Moreover, it appears that the developmental progression of human progenitor cells to more mature, lineage-defined cells proceeds normally from the NOD/SCID mouse BM to the peripheral hematopoietic tissues.
A novel finding in this study was that B cells could develop in mice from a CD34-enriched fraction that had been purged of CD19+ cells before transplanting. Whereas it could be argued that myeloid development may have occurred from committed progenitor cells (input fractions were contaminated with low levels of CD33+ cells), this data suggests that immature B cells developed de novo in the BM from CD34+ input cells. Analysis also revealed that CD19+ cells comprised a comparable fraction of huCD45+ cells in mouse tissues to that found in mice transplanted with unpurged CD34+ fractions. Furthermore, in all experiments, B cells were found to mature as they moved from the BM to the peripheral circulation. While CD19+ cells in BM were ≈75% to 85% negative for expression of surface IgM, those found in PB were 75% to 90% positive for expression of IgM. This shows that NOD/SCID mice are able to support human B-cell development to the stage of cell surface Ig expression from progenitors that lack surface expression of B lineage antigens.
Human CD14+ cells were found in BM, spleen, and PB for the duration of the engraftment period in all experiments (up to 15 weeks). This suggests that short-term myeloid progenitors continued to repopulate the hematopoietic tissues throughout this period of time. In vitro data also indicates that input cells able to produce CFUs were not depleted. Instead, evidence is presented that shows cells capable of giving rise to GM, BFU-E, and GEMM colonies in vitro, persist after engraftment of CD34+ cells for 10 weeks. Taken together, these data indicate that the NOD/SCID mouse provides an environment suitable for continuous generation of human myeloid lineages over extended periods of time. Additionally, most myeloid cells and short-term progenitors have a short half-life. Thus, the continued presence of both myeloid cells and colony-forming cells for extended engraftment periods suggests the graft is being maintained by a primitive cell capable of extensive proliferation. Several lines of evidence support the idea that the SRC is a primitive cell with engraftment and proliferative characteristics similar to those observed in the present study.29 This report further characterizes the SRC by presenting evidence that SRCs are contained in the CD34+ cell population and provides a basis for analyzing highly purified CD34+ cell subfractions for a more refined definition of the SRC.
Likewise, repopulating SRCs with the potential to home to and repopulate a secondary recipient are maintained in NOD/SCID mouse BM for at least 4 months. Engraftment levels in secondary recipients were not as high (range, 6% to 23% in BMs) as those generally found in primary recipients, however, this may be explained by the fact that human cells in the input fraction had to compete with normal mouse BM cells for available sites in the recipients' BM. Engrafted human cells were once again capable of producing myeloid and lymphoid populations, as well as sustaining a CD34+ population for the duration of the experiments (an additional 2 months). In an elegant experiment, Nolta et al16 genetically marked input human CD34+ cells derived from BM and PB and showed that BNX mice retained multilineage repopulating cells for up to 10.5 months. In the present experiments, we show that repopulating SRCs from CB, contained in the CD34+ cell fraction, are maintained in NOD/SCID marrow and retain their capacity to home to a second marrow following serial transplantation. These results suggest that the NOD/SCID model may be a useful assay for long-term repopulating cells contained in rare cell populations.
ACKNOWLEDGMENT
We gratefully acknowledge the invaluable assistance of Karen Helm for cell sorting and consultation on flow cytometric analysis. We would also like to thank Doreen Jumbeck of the National Jewish Center Biological Resource Center for excellent care and monitoring of experimental animals. Finally we thank Drs David Gordon and Bill Wood for providing human-specific primer sequences for the Cart-1 gene in advance of publication and Dr Ralph Quinones for comments on the manuscript.
Supported in part by Grants No. P-20 CA66207 (to C.J.H.), RO1 CA6508 (to E.J.S.), A130389 (to L.D.S.), and a Cancer Center Core grant (CA 46934) from the National Institutes of Health and/or National Cancer Institute, Bethesda, MD.
Address reprint requests to Christopher J. Hogan, PhD, Division of Medical Oncology, University of Colorado Health Sciences Center, Box B-171, 4200 E Ninth Ave, Denver, CO 80262.