Abstract
In recent studies we have shown that the expression of stem cell factor (SCF ) in human endothelial cells is regulated by inflammatory processes. Gram-negative bacteria, interleukin-1 (IL-1), and lipopolysaccharide were able to upregulate the expression of SCF in human umbilical vein endothelial cells (HUVEC) (Blood 83:2836, 1994). Interestingly enough c-kit, the receptor of SCF, is coexpressed on HUVEC, suggesting an autoregulatory mechanism. To investigate the relation of c-kit and inflammatory processes we stimulated HUVEC with IL-1α and we established an in vitro model of inflammation. Binding experiments with 125I-SCF were performed to study the c-kit receptor expression on HUVEC. Scatchard analysis revealed both high-affinity receptors (Kd ≈0.36 nmol/L) and low-affinity receptors (Kd ≈2.9 nmol/L). Exposure to IL-1α led to a significant 50% reduction of c-kit high-affinity receptors, whereas the number of low-affinity receptors was not affected, in comparison to a control group of untreated HUVEC. Furthermore, using Northern blot analysis we studied the regulation c-kit mRNA expression in HUVEC after stimulation with IL-1α. Kinetic experiments showed a time-dependent downregulation of c-kit specific transcripts. In addition, we cocultured HUVEC with diverse bacterial strains. Experiments were performed over time with 1 × 106 bacteria/mL. Our data showed that, in contrary to the previously reported upregulation of SCF mRNA expression, stimulation with Yersinia enterocolitica or with Neisseria meningitidis led to a significant time-dependent downregulation of c-kit mRNA within 3 hours. These data indicate that inflammatory stimuli such as IL-1 or living bacteria activate a mechanism that downregulates c-kit receptor expression in human endothelial cells during the state of inflammation.
THE c-kit PROTO-ONCOGENE encodes a transmembrane receptor with tyrosine-kinase activity1 that is structurally related to the platelet-derived growth factor receptor (PDGFR) and the macrophage colony-stimulating factor receptor (M-CSFR, c-fms), all members of the growth factor receptor family.2 The c-kit receptor protein has been identified as the natural receptor for a new cytokine, stem cell factor (SCF ),3,4 also known as kit ligand, Steel factor, or mast cell growth factor (MGF ). SCF and its receptor play a pleiotropic role in early hematopoiesis,5,6 melanogenesis,7 gametogenesis,7 and mast cell development.8 SCF acts in synergy with other hematopoietic growth factors, such as granulocyte colony-stimulating factor (G-CSF ), granulocyte-macrophage-CSF (GM-CSF ), interleukin-3 (IL-3), IL-1, IL-6, IL-7, and erythropoietin (EPO), on the maturation of hematopoietic stem cells and intermediate progenitor cells.5,6,8 The c-kit receptor has been shown to be highly expressed on hematopoietic progenitor cells,9 and has been found on acute myeloid leukemia (AML) blasts10 and some solid tumor cell lines.11 Both stem cell factor and c-kit receptor are expressed in small cell lung cancer cells.12
It has also been shown that SCF and its receptor are expressed in human endothelial cells.13,14 We have recently reported that the expression of SCF in human umbilical vein endothelial cells (HUVEC) is upregulated by a number of inflammatory mediators, such as IL-1α, bacterial lipopolysaccharide (LPS), and living bacteria.15 We also showed elevated SCF serum levels in patients with bacterial or viral diseases or rheumatoid arthritis.16 Furthermore, prophylactic in vivo administration of SCF has been shown to be protective in group B streptococcus-infected rats.17 These data suggest a potential role of this cytokine in acute inflammatory processes. Endothelial cells are also a major source for CSFs.18-21 The combination of CSFs together with other inflammatory cytokines and adhesion molecules derived from endothelial cells regulates the maturation and accumulation of effector cells at the site of inflammation.22 23 The role of the c-kit proto-oncogene product in inflammation is not known. To further clarify the physiologic significance of SCF during the inflammatory response, we studied the regulation of c-kit receptor expression in HUVEC in response to inflammatory stimuli. In particular, the potential effects of IL-1α and of various gram-negative bacteria as in vitro models of inflammation on c-kit expression in human endothelial cells were examined.
Our results complement those obtained with investigations concerning the regulation of SCF in HUVEC. These data may provide new insights in the physiologic significance of SCF and its receptor during the inflammatory response, suggesting an important role of their coexpression in human endothelial cells.
MATERIALS AND METHODS
Human endothelial cell cultures.HUVEC were prepared as previously described,24 using collagenase treatment (type I; GIBCO, Eggenstein, Germany), 0.01% in phosphate-buffered saline (PBS) for 30 minutes at 37°C, 5% CO2. Collected cells were centrifuged, and resuspended in RPMI 1640/M199 (1:2) (Flow Laboratories, Meckenheim, Germany) containing 1% penicillin/streptomycin, 1 mmol/L L-glutamine (GIBCO), and 10% heat-inactivated human AB serum (Department of Transfusion Medicine, Medical School Hannover, Hannover, Germany). Cells were seeded into tissue-culture flasks (Bibby-Corning, Stone, UK), coated with 0.1% gelatin (Sigma, München, Germany). After 4 to 5 days cells were grown to confluence and were passaged in a 1:2 split ratio. Characterization of endothelial cells was performed by von Willebrand factor expression using the alkaline-phosphatase–anti-alkaline-phosphatase (APAAP) staining method. When 80% confluence was reached, cells of the third or fourth passage were used for stimulation experiments. Unstimulated endothelial cells were used as controls.
Cell lines.The very well-characterized, spontaneously transformed human endothelial cell line ECV 30425,26 (European Collection of Animal Cell Cultures, Salisbury, UK) was grown to confluence in RPMI 1640/M199 (1:2) (Flow Laboratories) containing 1% penicillin/streptomycin, 1 mmol/L L-glutamine (GIBCO), and 5% fetal calf serum (FCS; GIBCO) at 37°C, 5% CO2. Cells were passaged in a 1:10 split ratio and used for stimulation when they reached 80% confluence.
The factor-dependent human megakaryoblastic leukemia cell line MO7e was cultured in RPMI 1640 supplemented with 1% penicillin-streptomycin (GIBCO), 1 mmol/L L-glutamine (GIBCO), 10% FCS, recombinant human (rh) GM-CSF (100 U/mL; Amgen, Thousand Oaks, CA), and rhIL-3 (100 U/mL; Amgen). The cell line was used as positive control for c-kit mRNA expression.
The human monomyelocytic cell line HL-60 was cultured in RPMI 1640 supplemented with 1% penicillin-streptomycin (GIBCO), 1 mmol/L L-glutamine (GIBCO), 10% FCS. Cells were used as negative control for c-kit expression in Northern blot analysis and Scatchard analysis.
Isolation of CD34+ cells.A healthy volunteer was treated 5 consecutive days with rhG-CSF (Amgen) at a concentration of 3 μg/kg/d. On day 6 after starting G-CSF treatment peripheral blood was drawn and CD34+ cells were obtained from peripheral stem cell harvesting with the CS-3000 cell separator (Baxter, Unterschleiβheim, Germany) followed by CD34+ enrichment with the Ceprate SC cell concentrator system using a biotin-labeled anti-CD34 monoclonal antibody (MoAb) and an avidin affinity column (CellPro Europe, Wezembeek, Belgium).
Recombinant factors.Recombinant IL-1α (IL-1α) was used in a concentration of 10 ng/mL (specific activity 2 × 107 U/mg) (this concentration was examined in previous studies to be optimal for stimulation15 ). This cytokine was kindly provided by Dr H. Radeke (Department of Molecular Pharmacology, Medical School Hannover). rhSCF was a gift from Dr K.M. Zsebo (Amgen).
Bacterial strains.Bacterial strain Yersinia enterocolitica, serotype 0:9 (Y enterocolitica 0:9) and bacterial strain Neisseria meningitidis B (N meningitidis B) were kindly provided by Dr M. Frosch (Department of Medical Microbiology, Medical School Hannover). All bacteria were used at a concentration of 1 × 106 bacteria/mL,15 respectively.
RNA preparation and Northern blot analysis.Total cellular RNA was extracted as described previously.27 Briefly, stimulated human endothelial cells were scraped from tissue-culture flasks, pelleted, and lysed in 5 mol/L guanidium isothiocyanate (Fluka, Neu-Ulm, Germany). Lysates were centrifuged through a cushion of 4.95 mol/L cesium chloride (Sigma, München, Germany). Ten milligrams of RNA per lane were size-fractionated by electrophoresis in a 1.2% agarose gel containing 0.7 mol/L formaldehyde, transferred to nylon membranes (Hybond N; Amersham, Braunschweig, Germany) by capillary suction overnight, and crosslinked by UV-light (Stratagene, La Jolla, CA). The cDNA probe for SCF (kindly provided by Dr K.M. Zsebo), a 455-bp SCF insert in pGEM3 vector, digested with Sac I, was labeled with 32phosphate-cytidine-triphosphate (32P-CTP; 3,000 Ci/mmol; Amersham) by in vitro transcription with sP6-RNA-polymerase (Promega, Woods, WI). The cDNA probe for c-kit (American Type Culture Collection, Rockville, MD), a 1,250-bp c-kit insert subcloned in pBS KS+ II (kindly provided by Dr K.-W. Sykora, Department of Pediatric Hematology and Oncology, Medical School Hannover) and digested with AvaII, resulting in a 666-bp c-kit insert, was labeled with 32P-CTP (3,000 Ci/mmol; Amersham) by in vitro transcription with T3-RNA-polymerase (Promega). As a control gene we used a cDNA probe for glyceraldehyde-phosphate-dehydrogenase (GAPDH; kindly provided by Dr R. Hipskind, Department of Molecular Biology, Medical School Hannover), a 1,270-bp insert in pBS KS+ linearized with EcoRI and labeled with 32P-CTP by in vitro transcription with T7-RNA-polymerase (Promega). Hybridization was performed for 16 hours at 58°C in 1× Denhardt's solution, 4× salt saturated citrate (SSC), and 50% deionized formamide. Membranes were washed in 2× SSC, 0.1% sodium dodecyl sulfate (SDS) at 58°C for 30 minutes, in 0.1× SSC, 0.1% SDS at 65°C for 20 minutes, respectively. A final wash was performed in 0.1× SSC, 0.1% SDS at 72°C for 10 minutes. Filters were exposed to Hyperfilm (Amersham) at −80°C.
Receptor-binding experiments.rhSCF was radiolabeled with 125iodine-monochloride (ICl; Amersham) according to the method described by Contreras et al.28 Briefly, 2 μg of rhSCF was added to a solution consisting of 1 mCi Na125I (New England Nuclear, Boston, MA), 0.2 mol/L sodium phosphate buffer (pH 7.2), and 0.02% Tween 20 (vol/vol), with a total volume of 50 μL. The iodination was started with 3 μL of 0.31 mmol/L ICl and was stopped after 90 seconds with addition of another 6 μL of ICl. Radioactive rhSCF was separated from free Na125I via a commercially available Sephadex G-25 column (NAP 5; Pharmacia, Freiburg, Germany). The eluted 125I-rhSCF was stored and stabilized with binding medium (1% bovine serum albumine [BSA], 0.1% sodium azide, 20 mmol/L HEPES in RPMI 1640/M199 [1:2], pH 7.2). HUVEC were detached using a solution containing 20 mmol/L HEPES, 150 mmol/L sodium chloride, and 500 nmol/L EDTA in PBS pH 7.2, centrifuged, and washed in PBS. To remove bound natural SCF (nSCF ) from cell surface, cells were treated for 20 seconds with sodium citrate buffer 10 mmol/L, pH 4.0), and subsequently washed with binding medium (1% BSA, 0.1% sodium azide, 20 mmol/L HEPES in RPMI 1640/M199 [1:2], pH 7.2). Binding experiments were performed on 2 × 106 HUVEC in binding medium with increasing concentrations of 125I-rhSCF ranging between 0.069 nmol/L and 2.2 nmol/L for 30 minutes at 18°C. Identical experiments were performed with and without the presence of unlabeled rhSCF (100-fold excess). Bound 125I-rhSCF und unbound 125I-rhSCF were separated by centrifugation of the cells through dibuthylphtalate/bis-2-ethyl-hexyl-phtalate (1:1.5). Pellets (bound radioactivity) and supernatants (free radioactivity) were quantitated in a gamma-counter (Packard, Frankfurt, Germany), respectively. Each experiment was performed with a single batch of HUVEC. Each data point was performed in triplicate. The number of binding sites per cell and dissociation constant were determined by Scatchard analysis.29
Flow cytometry analysis.CD34+ cells were analyzed directly after stimulation with IL-1α (10 ng/mL) or LPS (100 ng/mL15 ). Cells were fixed subsequently by the addition of 1% (vol/vol) formaldehyde in PBS for 30 minutes on ice. Cells were washed two times with fluorescence-activated cell sorting (FACS)-buffer (0.1% BSA and 0.1% sodium azide in PBS) and then resuspended in a diluted human Ig solution (10 mg/mL; GammaGard, Baxter) for blocking the Fc-receptors. Fluorescein isothiocyanate (FITC)-conjugated anti CD34 MoAb and phycoerythrine (PE)-conjugated anti-CD117 (both from Becton Dickinson, Heidelberg, Germany) were used for double-fluorescence analysis on CD34+ cells. FITC- and PE-labeled isotype antibodies (Immunotech, Hamburg, Germany) were used as controls. After this preincubation, cells were stained with the fluorochrome-labeled antibodies according to the manufacturer's instructions for 20 minutes on ice. ECV 304 and HUVEC, respectively, were detached from the surface of the culture flask by treatment with a solution containing 20 mmol/L HEPES, 150 mmol/L sodium chloride, and 500 nmol/L EDTA in PBS pH 7.2 for 10 to 20 minutes at 37°C. Cells were fixed and preincubated as described above, followed by incubation with unlabeled anti-CD117 (anti–c-kit) MoAb, clone 57A5D8B1 (kindly provided by Dr H.-J. Bühring, Tübingen, Germany), or a nonspecific control antibody for 30 minutes on ice. Cells were washed in FACS-buffer and subsequently incubated with PE-conjugated goat-antimouse Ig (Dako, Carpinteria, CA) according to the manufacturer's instructions. After staining, the cells were washed two times in FACS-buffer and analyzed on a FACScan flow cytometer (Becton Dickinson). FITC fluorescence was detected with a 530/30 nm, and PE fluorescence was detected with a 585/42 nm band pass filter. Cells were distinguished from debris and “machine noise” on the basis of their scatter profile. CD34+ cells were also gated on the basis of the scatter profile. Double-fluorescence staining showed more than 95% CD34+ cells in the gate.
RESULTS
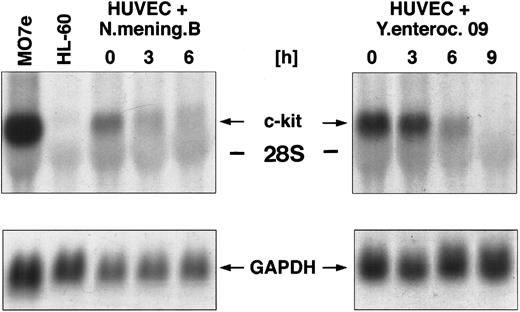
Regulation of c-kit mRNA after coculture of HUVEC with various gram-negative bacterial strains.The c-kit protein, the natural receptor for SCF, is expressed in human endothelial cells.13 To elucidate the potential effect of various inflammatory stimuli (eg, gram-negative bacteria) on the steady-state expression of c-kit mRNA, we cocultured HUVEC with Y enterocolitica 09 and N meningitidis, respectively (106 bacteria/mL), for different time periods. Total RNA of HUVEC was extracted and analyzed by Northern blotting for the regulation of c-kit mRNA expression. The results showed a maximal expression of the c-kit transcript at 5.5 kb in unstimulated HUVEC. Coculture of HUVEC with Y enterocolitica 09 resulted in a time-dependent downregulation of c-kit mRNA observed within 3 hours. Transcripts were not detectable after 6 hours of coculture (Fig 1). Similar results were obtained after coculture of HUVEC with N meningitidis B. Gene expression of c-kit was completely downregulated within 9 hours after coculture (Fig 1).
Northern blot analysis of c-kit mRNA expression in HUVEC cocultured with different gram-negative bacterial strains, namely Y enterocolitica and N meningitidis (106 bacteria/mL, respectively) for 0, 3, 6, and 9 hours. The membrane was then rehybridized with a probe for GAPDH.
Northern blot analysis of c-kit mRNA expression in HUVEC cocultured with different gram-negative bacterial strains, namely Y enterocolitica and N meningitidis (106 bacteria/mL, respectively) for 0, 3, 6, and 9 hours. The membrane was then rehybridized with a probe for GAPDH.
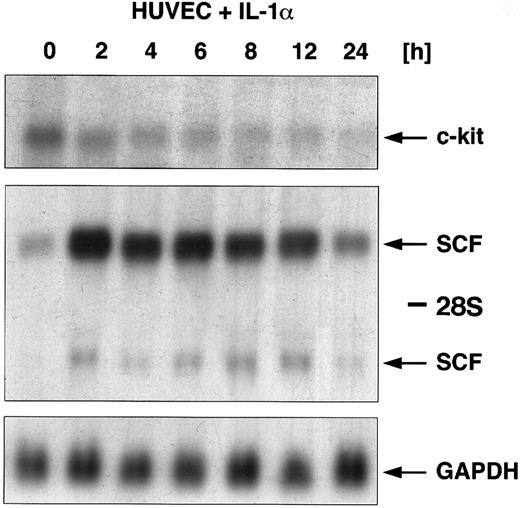
Regulation of c-kit and SCF mRNA by IL-1α.In addition to the coculture of HUVEC with gram-negative bacteria, HUVEC were stimulated with the classical inflammatory cytokine IL-1α. Figure 2 shows a Northern blot analysis for SCF and c-kit gene expression. HUVEC were exposed to IL-1α in kinetic experiments for a time period from 2 to 24 hours. Total RNA was extracted at each time point and the regulation of c-kit mRNA expression was examined using Northern blot analysis. Our data showed a maximal c-kit mRNA expression in unstimulated HUVEC. Exposure to IL-1α led to a downregulation of c-kit–specific transcripts. Kinetic experiments showed that IL-1α induced a time-dependent downregulation of c-kit mRNA occurring within 2 hours of stimulation and was almost not detectable anymore after 24 hours. In contrast to the downregulation of c-kit, SCF mRNA was upregulated in response to IL-1α. Accumulation occurred within 2 hours of stimulation with maximal expression after 4 hours, and returned to basal levels after 24 hours (see also ref 15). To demonstrate the counter-regulatory effects, we resume representative results for the regulation of SCF by IL-1α, which we have already published.15
Regulatory effects of IL-1α (10 ng/mL) on the expression of SCF mRNA and c-kit mRNA in HUVEC. Cells were stimulated with IL-1α over a time period from 0, 2, 4, 6, 8, 12, and 24 hours. Total cellular RNA was extracted and studied for regulation of both c-kit mRNA expression and SCF mRNA expression using Northern blot analysis. c-kit–specific mRNA could be detected at 5.5 kb (arrow); two SCF-specific transcripts are indicated by arrows (6.5 kb and 4.3 kb).The membrane was then rehybridized with a probe for GAPDH.
Regulatory effects of IL-1α (10 ng/mL) on the expression of SCF mRNA and c-kit mRNA in HUVEC. Cells were stimulated with IL-1α over a time period from 0, 2, 4, 6, 8, 12, and 24 hours. Total cellular RNA was extracted and studied for regulation of both c-kit mRNA expression and SCF mRNA expression using Northern blot analysis. c-kit–specific mRNA could be detected at 5.5 kb (arrow); two SCF-specific transcripts are indicated by arrows (6.5 kb and 4.3 kb).The membrane was then rehybridized with a probe for GAPDH.
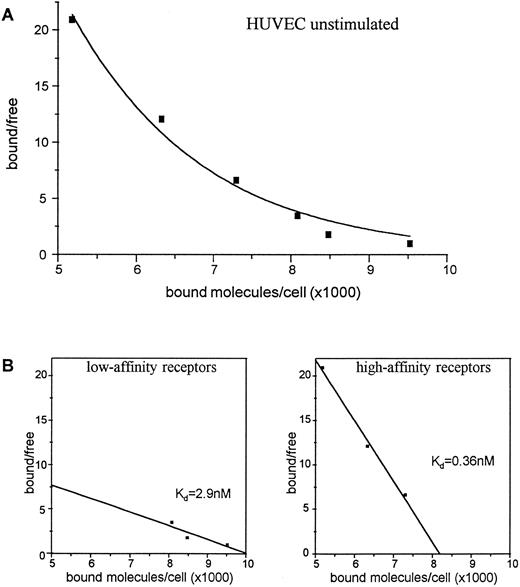
Expression of c-kit receptor protein on the cell surface of HUVEC.To investigate whether the IL-1α–induced regulation of c-kit mRNA was also reflected on the protein level, we performed receptor binding experiments for the presence and regulation of c-kit receptors on the cell surface of HUVEC using 125iodine (125I)-labeled SCF. The data showed that HUVEC display two types of SCF receptors: high-affinity receptors with a Kd of about 0.36 nmol/L and low-affinity receptors with a Kd of about 2.9 nmol/L. Scatchard analysis revealed about 8,200 high-affinity receptors per cell and about 10,000 low-affinity receptors per cell on unstimulated HUVEC (Fig 3).
Binding of 125I-SCF to unstimulated HUVEC (A). 2 × 106 cells were incubated with varying concentrations of 125I-SCF with and without 100-fold excess of unlabeled SCF at 18°C. Scatchard analysis of determination of c-kit receptors on unstimulated HUVEC (B). A representative experiment out of three is presented; each experiment used a single batch of HUVEC.
Binding of 125I-SCF to unstimulated HUVEC (A). 2 × 106 cells were incubated with varying concentrations of 125I-SCF with and without 100-fold excess of unlabeled SCF at 18°C. Scatchard analysis of determination of c-kit receptors on unstimulated HUVEC (B). A representative experiment out of three is presented; each experiment used a single batch of HUVEC.
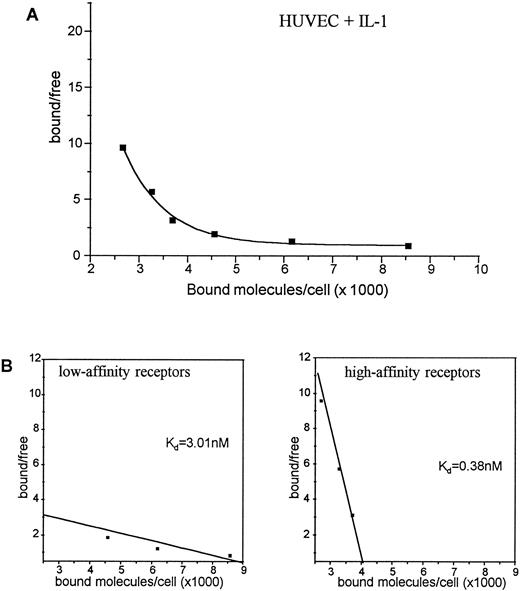
HUVEC exposed for 4 hours to IL-1α showed a significant and exclusive downregulation of about 50% of the high-affinity receptors to 4,100 copies per cell. In contrast, the low-affinity c-kit receptor expression was not affected by IL-1α (Fig 4). Extending the exposure to IL-1α up to 12 hours did not further affect the number of high-affinity receptors (data not shown). The data presented in Figs 3 and 4 reflect one representative experiment out of three, each performed with a single batch of HUVEC. The absolute number of copies per HUVEC varied dependent on the passage used for receptor binding experiments, but in all experiments stimulation with IL-1α led to the same percentage of high-affinity receptor reduction (data not shown).
Binding of 125I-SCF to HUVEC exposed for 4 hours to IL-1α (A). 2 × 106 cells were incubated with varying concentrations of 125I-SCF with and without 100-fold excess of cold SCF at 4°C. Scatchard analysis of c-kit receptors on HUVEC exposed for 4 hours to IL-1α (B). A representative experiment out of three is presented; each experiment used a single batch of HUVEC.
Binding of 125I-SCF to HUVEC exposed for 4 hours to IL-1α (A). 2 × 106 cells were incubated with varying concentrations of 125I-SCF with and without 100-fold excess of cold SCF at 4°C. Scatchard analysis of c-kit receptors on HUVEC exposed for 4 hours to IL-1α (B). A representative experiment out of three is presented; each experiment used a single batch of HUVEC.
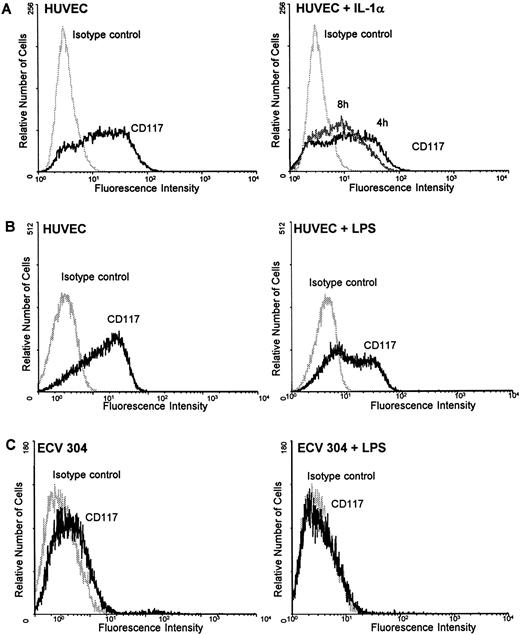
Regulation of c-kit protein by IL-1α and LPS on HUVEC, ECV 304, and CD34+ cells.To further clarify whether the mechanism of downregulating c-kit by inflammatory stimuli is a common mechanism or specific for endothelial cells during inflammation, HUVEC, ECV 304, a human transformed endothelial cell line, and CD34+ cells were examined for the regulation of c-kit surface protein by LPS using flow cytometry analysis. Incubation of HUVEC with the potent inflammatory stimuli IL-1α (10 ng/mL) for several time periods (4, 8, and 24 hours) resulted in a time-dependent downregulation of c-kit surface receptors with a maximum decrease after 8 hours (Fig 5A). After 24 hours receptor density returned toward basic levels (data not shown). Similar experiments with LPS (100 ng/mL) showed a detectable marked downregulation of c-kit protein expression after 24 hours in both HUVEC and ECV 304 (Fig 5B and C).
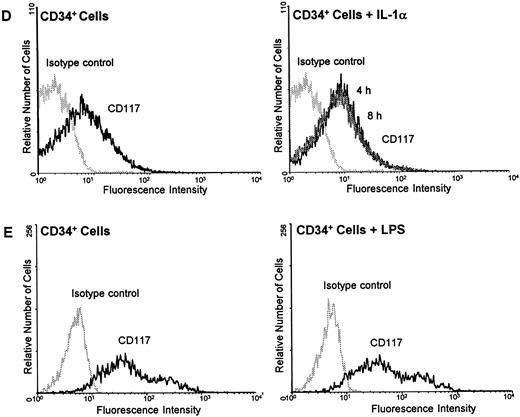
IL-1α and LPS downregulate c-kit receptor expression on the surface of HUVEC and ECV 304 (A through C) but not on CD34+ cells (D and E). Cells were stained using direct immunofluorescence with a PE-conjugated anti-CD 117 MoAb (anti–c-kit) (CD34+ cells) or indirect immunofluorescence using a PE-labeled secondary antibody (HUVEC, ECV 304). Background fluorescence (control) was measured using an irrelevant isotype FITC-conjugated antibody.
IL-1α and LPS downregulate c-kit receptor expression on the surface of HUVEC and ECV 304 (A through C) but not on CD34+ cells (D and E). Cells were stained using direct immunofluorescence with a PE-conjugated anti-CD 117 MoAb (anti–c-kit) (CD34+ cells) or indirect immunofluorescence using a PE-labeled secondary antibody (HUVEC, ECV 304). Background fluorescence (control) was measured using an irrelevant isotype FITC-conjugated antibody.
In contrast, neither IL-1α nor LPS influenced the c-kit receptor expression in CD34+ cells. Stimulation of the cells with either IL-1α or LPS for 4, 8, or 24 hours revealed no changes in c-kit protein on the cell surface as determined by flow cytometry analysis (Fig 5C and D).
DISCUSSION
The c-kit receptor protein plays a pleiotropic role in early hematopoiesis,9 melanogenesis,7 and gametogenesis.7 Stromal cells, including endothelial cells, display the c-kit receptor protein, as shown in previous studies.13 14
Recently, we were able to show that the natural ligand for this receptor, SCF, which is coexpressed in these cells,13 is regulated by a number of inflammatory stimuli in freshly isolated HUVEC and in endothelial cells of the transformed phenotype (ECV 304).15
In the present study we examined the role of human c-kit receptors in the inflammatory response using an in vitro model of inflammation with living bacteria as well as with IL-1α and LPS.
In this model we investigated the regulation of c-kit receptor expression in coculture experiments of HUVEC with various gram-negative bacterial strains after stimulation with IL-1α or LPS. The results presented here indicate that the c-kit receptor is also involved in the regulation of inflammatory processes.
Two gram-negative bacterial strains used in this in vitro model of inflammation (Y enterocolitica and N meningitidis, respectively) were able to induce a significant time-dependent decrease of c-kit mRNA expression after coculture with HUVEC (Fig 1). As we showed in earlier studies, the same experimental design yielded an upregulation of mRNA for SCF.15 Similar to the bacterial strains that were able to induce these counter-regulatory effects, IL-1α, one of the pivotal cytokines in the management of inflammation, was found to downregulate c-kit–specific transcripts in HUVEC within 24 hours (Fig 2). IL-1α has been previously shown to induce a significant upregulation of SCF mRNA expression as well as elevated SCF protein levels in the supernatant of HUVEC.15 The regulation of SCF expression in HUVEC after exposure to IL-1 is controversially discussed in recent publications,13,14,30 where Aye et al13 and Buzby et al 30 showed an induction of SCF mRNA by 5 to 20 U/mL IL-1β, whereas Heinrich et al14 showed no induction by 750 U/mL IL-1α. The data presented in our study about the regulation of c-kit mRNA expression correspond to those obtained from Buzby et al30 for short time stimulation with IL-1β. In contrast to the data of this group, who found c-kit mRNA expression returned to basal levels after 24 hours, our data showed a continuous downregulation of c-kit transcripts within 24 hours after stimulation with IL-1α (Fig 2).
Receptor binding experiments using 125I-SCF showed the existence of high-affinity and low-affinity receptors in HUVEC with similar binding affinities as demonstrated by Broudy et al.31 Exposure to IL-1α for 4 hours led to a significant downregulation of the high-affinity receptors for c-kit on the endothelial cell surface (Fig 4), whereas the number of the low-affinity receptors were not affected by IL-1α. Examples of other growth factor receptors32 showed that affinity changes can be achieved through noncovalent dimerization of different receptor subunits. A similar mechanism might be the case for c-kit, with the difference being that the two subunits are identical and thus the high-affinity receptor would be a product of receptor dimerization, while a conformational change to a monomeric molecule might reflect a low-affinity receptor.33 A possible explanation of our observations could be that the relational expression of high-and low-affinity receptors in unstimulated endothelial cells is a product of a balanced steady state, where a transient suppression of the c-kit mRNA would lead primarily to a diminished expression of high-affinity receptors only, as it is shown here. Attempts to obtain further evidence supporting this theory have led to ongoing experiments.
The concomitant decrease of c-kit mRNA and receptor protein downregulation leads to the assumption that last-mentioned is a result of diminished mRNA availability for translation. Another possible explanation could be a mechanism of increased c-kit receptor breakdown.
In endothelial cells (primary HUVEC as well as cells of the transformed phenotype), the expression of the c-kit receptor by infectious or inflammatory stimuli seems to be differently regulated as compared to CD34+ cells (Fig 5A through E). Although c-kit was downregulated in HUVEC and ECV 304, no effect could be observed in similar experiments with CD34+ cells, which are bearing a high number of c-kit receptors on their cell surface. In an earlier observation SCF protein was found to be enhanced in IL-1–stimulated supernatants of endothelial cells.15 From these data we suggest that during the state of inflammation SCF does not bind to the endothelial surface anymore (autocrine loop), but is now available as a hematopoietic growth factor for CD34+ cells.
Endothelial cells are among the most important cells of the inflammatory response. The production of numerous cytokines, including hematopoietic growth factors such as G-CSF and GM-CSF,20 and the expression of adhesion molecules by activated endothelial cells contribute to the regulation of production of effector cells as well as their accumulation at the site of inflammation.
In summary, the data presented here show an interaction of diverse inflammatory stimuli with SCF and its receptor c-kit in human endothelial cells. In earlier studies SCF has been shown to stimulate synergistically with other hematopoietic growth factors the proliferation and maturation of progenitor cells,5,6,8 including enriched CD34+ cells.34
Inflammatory stimuli activate SCF expression as part of an acute cytokine response15 and downregulate c-kit expression, as shown in this report. This downregulation of the receptor protein might indicate a temporarily arrest of an autocrine mechanism to maintain a steady state of SCF for hematopoietic homeostasis.
Previously it has been shown that endothelial cells display a soluble form of the c-kit receptor protein.31 Therefore, downregulation of c-kit during the state of inflammation could also reflect the release of the protein from the cell surface of the endothelial cell into the serum and act as a local regulator of SCF.
ACKNOWLEDGMENT
We are grateful to Marlene Reuter and Nicole Wittner for excellent technical assistance.
Supported in part by grants from the Deutsche Forschungsgemeinschaft (SFB 244).
Address reprint requests to Andrea König, PhD, Laboratory of Leukocyte Biology, Memorial Sloan-Kettering Cancer Center, 1275 York Ave, PO Box 320, New York, NY 10021.