Abstract
The binding of late stage erythroid cells to fibronectin (FN) has been well characterized and is believed to be critical for the terminal stages of erythroid differentiation, but the adhesive properties of more primitive murine erythroid progenitors and the role of these interactions during earlier stages of erythropoiesis has not been determined. Using chymotryptic fragments and inhibitory probes, we have tested the ability of each of the major cell binding domains of FN; the RGDS sequence, the CS-1 sequence, and the carboxy-terminal heparin-binding domain (HBD), to promote adhesion of primitive burst-forming unit-erythroid (BFU-E), mature BFU-E, and colony-forming unit-erythroid (CFU-E). We found that only 10% to 15% of BFU-E bound to FN or to the RGDS sequence in contrast to 75% to 85% of CFU-E. Approximately 50% to 70% of BFU-E and 60% to 80% of CFU-E bound to the carboxy-terminal HBD and to the CS-1 sequence. The binding of BFU-E and CFU-E to the RGDS and CS-1 sites was blocked by β1 integrin antibodies. These results suggest that binding to FN determinants is developmentally regulated during early erythroid differentiation. Erythroid progenitor migration within the bone marrow is thought to be important for the eventual release of reticulocytes into the circulation. A correlation between FN binding and the migratory capacity of erythroid cells has been suggested, although the ability of FN to promote migration of erythroid progenitors has not been directly measured. We measured migration of CFU-E on fragments of FN containing each cell binding region. CS-1–containing fragments, in addition to promoting adhesion of both BFU-E and CFU-E, supported the highest levels of CFU-E migration (11-fold above background). Migration was sixfold above background on intact FN and only threefold above background on RGDS-containing fragments. Fragments containing HBD alone, although they promoted adhesion of CFU-E, failed to support significant levels of migration. These results show that specific domains of FN possess distinct adhesion- and migration-promoting properties for murine erythroid progenitors. Regulation of the adhesive properties during erythroid differentiation may alter the ability of progenitors to migrate in the bone marrow and thus play an important role in normal murine erythroid differentiation.
BONE MARROW stromal cells synthesize growth factors, cell adhesion molecules, and extracellular matrix (ECM) molecules which are essential for the establishment of a microenvironment supportive of hematopoietic cell growth and differentiation.1 Fibronectin (FN), a multifunctional ECM glycoprotein found in the bone marrow,2 has been shown to influence the adhesion, migration, growth, and differentiation of many cell types, including hematopoietic cells.3 There are three major cell binding regions of the FN molecule which serve as ligands for distinct cell surface receptors. Adhesion to the Arg-Gly-Asp-Ser (RGDS) sequence in the central region of the FN molecule4 is mediated by the α5β1 very late antigen antigen-5 (VLA-5) integrin receptor.5 The alternatively-spliced type III connecting segment (IIICS) region of FN contains two cell binding sites. The major site is located within the first 25 amino acids (CS-1) of the IIICS region.6,7 A second less active site is the REDV amino acid sequence present in the CS-5 peptide of the IIICS region.6,8 Adhesion to both the REDV and LDV sequences is mediated by the α4β1 (VLA-4) integrin.9-11 In addition, at least three distinct peptide sequences within the carboxy-terminal heparin binding domain (HBD) promote cell binding mediated by cell surface chondroitan sulfate proteoglycans (CSPG) or the cooperative interactions of VLA-4 and CSPG.12-14
Interactions of erythroid cells with FN are believed to be essential for erythropoiesis, particularly for the terminal stages of erythroid differentiation.15-19 The erythroid progenitor compartment is composed of three distinct epo-responsive populations of cells; the primitive burst-forming unit-erythroid (BFU-E), mature BFU-E, and the colony-forming unit-erythroid (CFU-E) progenitor cells, which represent successively mature populations along the erythroid pathway.20 Murine erythroleukemic cell lines and normal human progenitors at the CFU-E stage of development express both VLA-4 and VLA-5 integrins, and binding to FN was found to be developmentally regulated during the later stages of erythroid differentiation from CFU-E to the mature red blood cell.21-27 The binding properties of early stage erythroid cells is less well documented. Reports on human BFU-E binding have shown either low level or lack of adhesion to FN,17 28-30 while there have been no reports on the binding of murine primitive BFU-E or mature BFU-E to FN.
The role of cell adhesion to FN during erythroid differentiation has not been determined, although studies have suggested that FN is necessary both in vitro and in vivo to provide the appropriate niche for erythroid development and possibly also provide a proliferative stimulus for erythroid cells.15-18 During erythroid development, progenitors are believed to migrate within the bone marrow toward the venous sinuses to enable the eventual release of reticulocytes into the general circulation.31-33 A correlation between the migratory capacity of erythroid cells and their adherence to FN has been suggested,17 26 although the ability of FN determinants to promote the migration of erythroid progenitors has not been directly examined. In the present studies, we have characterized the binding of normal murine primitive BFU-E, mature BFU-E, and CFU-E to each cell binding domain of FN. We then examined the ability of each region of FN to promote migration of CFU-E. Our data suggest that there are distinct erythroid cell adhesion and migration promoting regions of FN. Developmental changes in erythroid cell binding to specific domains of FN may lead to changes in their ability to migrate within the marrow microenvironment and may be important for normal erythropoiesis.
MATERIALS AND METHODS
Preparation of substrates and synthetic peptides.FN was purified from human plasma (Lifesource, Glenview, IL) as previously described,34 except that the gelatin-agarose column was washed with 1 mol/L urea before elution of FN with 4 mol/L urea. The chymotryptic cell binding domain (CBD) and heparin-binding fragments of FN were purified as previously described.34 35 Three major heparin-binding fragments (30 kD, 35 kD, and 42 kD) were obtained in the 1 mol/L NaCl eluate from a heparin-agarose column. To separate these heparin-binding fragments, the 1 mol/L NaCl eluate was passed through a diethyl aminoethyl sepharose column (Pharmacia Fine Chemicals, Uppsala, Sweden). The 30- and 35-kD fragments were collected in the unbound fraction, while the 42-kD fragment was eluted from the column with 100 mmol/L NaCl. All three heparin-binding polypeptides possess identical N-terminal amino acid sequences (VQTAVTAIPAP), initiating at the amino acid 1625 in the human plasma FN sequence (GenBank accession no. P02751), confirming the origin of these polypeptides from the carboxy-terminal end of the FN molecule (Fig 1). 2% FN-free bovine serum albumin (BSA) was prepared by passing a 2% BSA solution (Sigma Chemical Co, St Louis, MO) in 10 mmol/L NaPO4 containing 150 mmol/L NaCl pH 7.5 phosphate-buffered saline (PBS) over a gelatin-agarose affinity column. Crude synthetic peptides CS-1, CS-2, CS-3, (Fig 1) were synthesized by Vega Biotechnologies Inc, Tucson, AZ. These peptides were purified by reverse-phase high-performance liquid chromatography using a 2.2 × 25 cm Vydac C4 column (15 to 20 mm particle size, 300 Å pore size; The Nest Group, Southboro, MA) and a 0% to 60% acetonitrile gradient. The synthetic peptide PREDVDY was synthesized and purified by Dr Peter Kim at the Whitehead Institute, Cambridge, MA. Purified synthetic peptides GRGDSP and GRGESP were obtained from Calbiochem Corp, La Jolla, CA. Collagen type IV and laminin were purchased from Collaborative Research (Bedford, MA), and Vitrogen (Collagen types I and III) was purchased from the Collagen Corp (Palo Alto, CA).
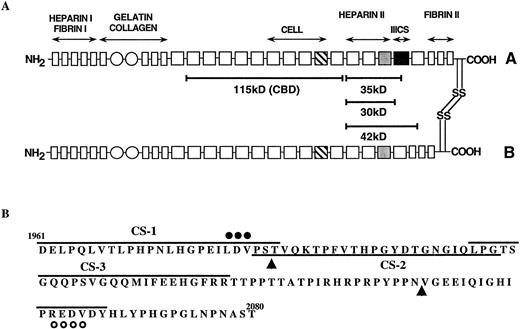
(A) A model of human plasma FN showing two heterologous subunits (A and B), which are linked at their carboxy-termini by two disulfide bonds. Each subunit contains a series of type I (rectangles), type II (circles), and type III (squares) homologous repeats. The type III repeats which contain the classical RGDS cell adhesion sequence (▧) and heparin-binding cell adhesion sequences () are shown. The alternatively spliced type III connecting segment (IIICS), which contains two known cell adhesion sequences is indicated (▪). The location of the chymotryptic fragments used in these studies, along with their approximate molecular weights, are shown by solid bars. Antibody recognition and fibrin-binding data suggest that the 42-kD fragment originates from the B chain, while the 30-/35-kD fragments are from the A chain. (B) Amino acid sequence of the type IIICS region, which spans amino acids 1961-2080 of the A chain of human plasma FN. Synthetic peptides used in these studies (CS-1, CS-2, CS-3, and PREDVDY) are indicated by solid lines. The two sequences known to be active in cell adhesion are indicated by closed circles (LDV) and open circles (REDV). Arrowheads indicate putative splice sites in the IIICS region.
(A) A model of human plasma FN showing two heterologous subunits (A and B), which are linked at their carboxy-termini by two disulfide bonds. Each subunit contains a series of type I (rectangles), type II (circles), and type III (squares) homologous repeats. The type III repeats which contain the classical RGDS cell adhesion sequence (▧) and heparin-binding cell adhesion sequences () are shown. The alternatively spliced type III connecting segment (IIICS), which contains two known cell adhesion sequences is indicated (▪). The location of the chymotryptic fragments used in these studies, along with their approximate molecular weights, are shown by solid bars. Antibody recognition and fibrin-binding data suggest that the 42-kD fragment originates from the B chain, while the 30-/35-kD fragments are from the A chain. (B) Amino acid sequence of the type IIICS region, which spans amino acids 1961-2080 of the A chain of human plasma FN. Synthetic peptides used in these studies (CS-1, CS-2, CS-3, and PREDVDY) are indicated by solid lines. The two sequences known to be active in cell adhesion are indicated by closed circles (LDV) and open circles (REDV). Arrowheads indicate putative splice sites in the IIICS region.
Isolation of murine bone marrow cells.Bone marrow cells were collected from the femurs and tibiae of 5- to 9-week-old male C57BL6 mice (Jackson Laboratories, Bar Harbor, ME, Harlan Sprague Dawley Inc, Indianapolis, IN) by flushing the medullary cavity with Iscove's modified Dulbecco's medium (IMDM) (Hazelton Inc, St Lenexa, KS) supplemented with 5% decomplemented fetal bovine serum (FBS; GIBCO Laboratories, Grand Island, NY) using a 25G5/8″ needle and syringe. Nucleated marrow cells were obtained from the interface after Histopaque Ficoll 1119 (Sigma Chemical Co) separation. Following a plastic adherence step, nonadherent cells were collected and used as a source of erythroid progenitors.
Erythroid progenitor cultures.The presence of CFU-E, mature BFU-E, and primitive BFU-E was detected using the assay conditions previously described.20 The presence of CFU-E was determined by incubating cells in IMDM containing 0.8% methylcellulose (MC) (Dow Chemical Co, Midland, MI), 1% deionized BSA, 44 mmol/L NaHCO3 , 10−4 mol/L β-mercaptoethanol, 0.9 mmol/L Imferon, and 30% decomplemented FBS (IMDM + MC) to which 1 U/mL human recombinant erythropoietin (epo; Amgen Biologicals, Thousand Oaks, CA) was added. After 48 hours at 37°C, hemoglobinized CFU-E colonies were detected by staining the cultures with a 0.2% (wt/vol) benzedine dihydrochloride solution.36 Colonies that were counted as CFU-E contained 4 to 30 tightly clustered erythroblasts that appeared blue under bright field microscopy after benzedine staining. A total of 40 to 70 CFU-E colonies were obtained per 50,000 cells under these culture conditions. Mature and primitive BFU-E were detected by incubating bone marrow cells in IMDM + MC containing 3 U/mL epo and WEHI-3 conditioned medium (as a source of interleukin-3 [IL-3]) at 37°C for 4 to 5 days and 11 days, respectively. Mature BFU-E were clusters of 1 to 3 hemoglobinized colonies that appear red under bright field microscopy. The primitive BFU-E were very large clusters of hemoglobinized colonies. Plating 2 × 105 unfractionated bone marrow cells gave rise to 15 to 30 mature BFU-E and 10 to 20 primitive BFU-E.
Cell adhesion assays.Plastic 24-well plates were obtained from Costar Corp (Cambridge, MA) and wells were coated with FN, fragments of FN, laminin, collagen types I and III, or collagen type IV. Except for Vitrogen, concentrated substrate stocks were diluted to the desired concentrations in sterile PBS and incubated in wells for 2 hours at room temperature (RT). Vitrogen stock was diluted in sterile 0.012N HCl solution and allowed to dry in the wells. The concentration of substrates used in these experiments was determined experimentally as the input concentration of protein that promoted maximal adhesion of erythroid progenitors: FN (16.7 μg/cm2 ), CBD (8.3 μg/cm2 ), 30-/35-kD fragments (4.4 μg/cm2 ), 42-kD fragment (3.2 μg/cm2 ), collagen types I and III (28 μg/cm2 ), collagen type IV (25 μg/cm2 ), and laminin (5 μg/cm2 ). Once wells were coated with the indicated substrates, 2% FN-free BSA was added for 15 minutes at RT. The wells were washed once with IMDM and 50,000 cells (CFU-E assays) or 200,000 cells (BFU-E assays) were added to each well. After 2 hours at 37°C, unattached cells were collected from each well. Each well was then washed twice with warm IMDM and the washes were added to the unattached fraction. Attached cells were either cultured directly on the substrates or detached from the substrates mechanically by pipetting as follows. All CFU-E assays were cultured directly on substrates except when assaying adhesion to 30-/35-kD fragments. All BFU-E assays and the CFU-E assays on 30-/35-kD fragments were performed after detaching the attached cells from the substrate. Background adhesion to the wells was measured by incubating cells in wells incubated with 2% FN-free BSA alone. The percent of cell adhesion to BSA or the substrates was calculated by dividing the number of attached progenitors by the total recovered from each well. Results are presented as the mean percent adhesion plus or minus the standard error of the mean (SEM).
Heparin, synthetic peptide, and antibody competition assays.In these assays, 48-well plates (Costar Corp) were coated with substrates and cells were applied to each well at the same input concentration per cm2 as described above. When measuring the effect of heparin on cell adhesion, a 50-mmol/L solution (200 μg/cm2 ) of heparin (Calbiochem) diluted in PBS was added to each substrate coated well after blocking with 2% FN-free BSA. Plates were then incubated for 15 minutes at RT, after which the wells were washed with IMDM to remove any unbound heparin. Marrow cells were then added as described above. When assaying for the effect of synthetic peptides (GRGDSP, GRGESP, CS-1, CS-2, CS-3, or PREDVDY) or antibodies, marrow cells were incubated on the substrates for 2 hours at 37°C in the presence of the indicated final concentration of peptide or antibody. Antibodies used in these studies include IgG purified from a rabbit antiserum recognizing the murine β1 subunit35 nonimmune rabbit IgG control antibody (Zymed Laboratories, South San Francisco, CA), R1-2 monoclonal antibody (a gift from I.L. Weissman, Stanford University, Palo Alto, CA), which recognizes the α4 integrin subunit.37
Erythroid progenitor migration assays.CFU-E migration assays were performed in 8-mm diameter blind well chambers (Neuro Probe Inc, Cabin John, MD) using 13 mm diameter polyvinylpyrrolidone-free polycarbonate (PC) filters (Poretics Corp, Livermore, CA) with a 5 μm pore size (see Table 2). Filters were coated with substrates on the bottom surface as described.38 Briefly, the filters were either floated on 0.5 mL of a 0.1 mol/L NaCO3 buffer pH 9.5 containing the desired concentrations of FN (50 μg/mL), CBD (25 μg/mL), 30/35-kD (12.5 μg/mL), 42-kD (10 μg/mL), or 45-kD gelatin-binding domain (GBD; 25 μg/mL). These coating concentrations were determined experimentally to promote maximal migration of CFU-E under these conditions (data not shown). Filters were also floated on 0.5 mL of 2% FN-free BSA as a negative control. After an overnight incubation at 37°C, the filters were rinsed 4 to 5 times in distilled water, then PBS, and air dried. Migration assays were performed in IMDM containing 1% deionized BSA, 44 mmol/L NaHCO3 , 10−4 mol/L β-mercaptoethanol, 0.9 mmol/L Imferon, 1× insulin-transferin-selenium (ITS-S supplement; GIBCO Laboratories), and 1 U/mL human recombinant epo (Amgen Biologicals) (migration assay medium). Bone marrow cells (obtained as described above) were resuspended in migration assay medium at a concentration of 8 × 105 cells/mL, and 0.6 mL of this cell suspension was added to the top of each blind well chamber. After 1 hour at 37°C, the cells in the top chamber were removed. The PC filters were collected and the bottom of the filter was assayed for the presence of CFU-E. Cells in the lower well of the blind well chamber were also collected and assayed for the presence of CFU-E. The results are presented as either migration index ± SEM (calculated by dividing the number of CFU-E migrated on a substrate-coated filter by the number of CFU-E migrated on an uncoated filter) or percent migration ± SEM (calculated by dividing the number of CFU-E migrated by the total input CFU-E). The number of migrated CFU-E is the sum of CFU-E found on the bottom of the filter and CFU-E found in the bottom well of the blind well chamber.
Effect of Heparin on Adhesion of Erythroid Progenitors to Heparin Binding Fragments of FN
| Substrate . | % Adhesion . | |||
|---|---|---|---|---|
| . | Heparin . | CFU-E . | Mature BFU-E . | Primitive BFU-E . |
| BSA | − | 6 ± 1 | <1 | <1 |
| n = 19 | n = 21 | n = 21 | ||
| 30/35 kD | − | 86 ± 3 | 66 ± 3 | 64 ± 4 |
| n = 9 | n = 17 | n = 14 | ||
| + | 85 ± 3 | 37 ± 4* | 44 ± 4* | |
| n = 9 | n = 17 | n = 14 | ||
| 42 kD | − | 65 ± 4 | 48 ± 3 | 44 ± 3 |
| n = 5 | n = 5 | n = 5 | ||
| + | 13 ± 3* | 7 ± 2* | <1* | |
| n = 5 | n = 3 | n = 3 | ||
| Substrate . | % Adhesion . | |||
|---|---|---|---|---|
| . | Heparin . | CFU-E . | Mature BFU-E . | Primitive BFU-E . |
| BSA | − | 6 ± 1 | <1 | <1 |
| n = 19 | n = 21 | n = 21 | ||
| 30/35 kD | − | 86 ± 3 | 66 ± 3 | 64 ± 4 |
| n = 9 | n = 17 | n = 14 | ||
| + | 85 ± 3 | 37 ± 4* | 44 ± 4* | |
| n = 9 | n = 17 | n = 14 | ||
| 42 kD | − | 65 ± 4 | 48 ± 3 | 44 ± 3 |
| n = 5 | n = 5 | n = 5 | ||
| + | 13 ± 3* | 7 ± 2* | <1* | |
| n = 5 | n = 3 | n = 3 | ||
Bone marrow cells were incubated in wells coated with untreated and heparin-treated 30-/35- and 42-kD heparin binding fragments. Unattached and attached populations were then assayed for the presence of eyrthroid progenitors. The results were expressed as the mean percent cell adhesion ± SEM.
P ≤ .002.
RESULTS
Erythroid progenitors attach to FN.The adhesion specificity of murine erythroid progenitors was examined by incubating freshly isolated bone marrow cells in wells coated with either purified FN, collagen type IV, laminin, or a mixture of collagen types I and III. After allowing cells to attach for 2 hours at 37°C, the attached and unattached populations of cells were recovered and assayed for the presence of erythroid progenitors. Data presented in Table 1 shows that 76% of CFU-E attached to FN, while only 11% of primitive BFU-E and 5% of mature BFU-E bound. Adhesion of CFU-E was dependent on the input concentration of FN applied to the wells, with half-maximal attachment at coating concentrations of 3 μg/cm2 and maximal attachment (shown in Table 1) at 16 μg/cm2. Adhesion of mature and primitive BFU-E was not increased at these or higher coating concentrations of FN (data not shown). All progenitors showed low but significant levels of binding to laminin. Ten percent to 15% adhesion was also observed on collagen type IV for mature BFU-E and CFU-E and on collagen types I and III for CFU-E. As a positive control, the same concentrations of all substrates supported 70% to 80% adhesion of Hepa 1 cells (Table 1). None of the erythroid progenitors attached significantly to wells coated with heat-inactivated BSA (control). The substrates were not toxic to erythroid progenitors during the adhesion assay, as the recovery of progenitors following adhesion assays (the unattached plus the attached populations) was comparable to the progenitors recovered from the BSA-coated wells (data not shown). To measure CFU-E binding, attached cells were simply overlaid with methylcellulose-containing medium as described. Resulting CFU-E colonies were easily scorable and the total recoveries were similar to those from BSA-coated dishes. Because of the dispersed morphology of the BFU-E colonies when cells were grown in contact with FN, it was impractical to score BFU-E using the overlay method. Therefore, for all BFU-E adhesion assays performed in this study, attached cells were mechanically detached from the dish, then plated in methylcellulose in a fresh dish as described. Using this detachment method, the number of BFU-E recovered from coated dishes was comparable to that obtained from BSA-coated dishes and colonies were easily scorable. Because FN promoted the highest levels of adhesion, we next examined the ability of each cell binding site of FN to promote binding of erythroid progenitor cells.
Adhesion of Erythroid Progenitors to Extracellular Matrix Proteins
| Substrate . | % Adhesion . | |||
|---|---|---|---|---|
| . | CFU-E . | Mature BFU-E . | Primitive BFU-E . | HEPA1 . |
| BSA | 6 ± 1 | <1 | <1 | <5 |
| n = 19 | n = 21 | n = 21 | n = 3 | |
| Fibronectin | 76 ± 2* | 5 ± 2* | 11 ± 2* | 83 ± 5* |
| n = 19 | n = 13 | n = 16 | n = 3 | |
| Laminin | 16 ± 1* | 12 ± 2* | 12 ± 4* | 69 ± 4* |
| n = 4 | n = 7 | n = 7 | n = 3 | |
| Collagen types I and III | 13 ± 3 | <5 | <1 | 78 ± 7* |
| n = 4 | n = 2 | n = 2 | n = 3 | |
| Collagen type IV | 15 ± 2* | 13 ± 3 | <5 | 82 ± 3* |
| n = 7 | n = 2 | n = 2 | n = 3 | |
| Substrate . | % Adhesion . | |||
|---|---|---|---|---|
| . | CFU-E . | Mature BFU-E . | Primitive BFU-E . | HEPA1 . |
| BSA | 6 ± 1 | <1 | <1 | <5 |
| n = 19 | n = 21 | n = 21 | n = 3 | |
| Fibronectin | 76 ± 2* | 5 ± 2* | 11 ± 2* | 83 ± 5* |
| n = 19 | n = 13 | n = 16 | n = 3 | |
| Laminin | 16 ± 1* | 12 ± 2* | 12 ± 4* | 69 ± 4* |
| n = 4 | n = 7 | n = 7 | n = 3 | |
| Collagen types I and III | 13 ± 3 | <5 | <1 | 78 ± 7* |
| n = 4 | n = 2 | n = 2 | n = 3 | |
| Collagen type IV | 15 ± 2* | 13 ± 3 | <5 | 82 ± 3* |
| n = 7 | n = 2 | n = 2 | n = 3 | |
Bone marrow cells were incubated in wells coated with the indicated substrates. The unattached and attached populations were then assayed for the presence of CFU-Es, mature BFU-Es, and primitive BFU-Es. Hepal cell adhesion assays were performed as previously described (Patel et al39 ). Briefly, the cells were metabollically labeled and incubated in wells coated with the indicated substrates. The ratio of adherent cpm to the input cpm was then calculated. All results are expressed as the mean percent cell adhesion ± SEM.
P ≤ .02.
Erythroid progenitors adhere to known binding sites of FN.Proteolytic fragments generated from human plasma FN have been used to localize cell adhesion-promoting activity to the central and the carboxy-terminal regions of the molecule. Previous studies have shown that fragments of FN can possess adhesion- and migration-promoting activities, which the intact molecule does not. To identify the site(s) of FN that mediate binding of erythroid progenitors, we first measured adhesion of CFU-E and BFU-E to FN fragments purified from a chymotryptic digest of human plasma FN (see Materials and Methods). The 115-kD CBD is derived from the central region of the FN molecule (Fig 1) and contains the tetrapeptide sequence RGDS. Three major heparin-binding fragments of 42, 35, and 30 kD are derived from the carboxy-terminal end of the molecule (Fig 1). The 42-kD fragment was separated from the 35- and 30-kD species by anion-exchange chromatography. The IIICS region of FN, which contains the CS-1 and REDV cell binding sequences, is adjacent to the carboxy-terminal HBD and is present, at least in part, in the 35-kD fragment, but not in the 42-kD fragment. The presence of the CS-1 peptide sequence in the 35-kD heparin-binding fragment was confirmed by immunoblotting with antibodies raised against the CS-1 synthetic peptide (Dwivedi and Patel, unpublished observations, September 1990). Based on the molecular weight of the fragment and the known amino terminal sequence, the CS-2 region and part of the CS-3 region are also included in the 35-kD fragment, while the PREDVDY sequence is most likely not present.
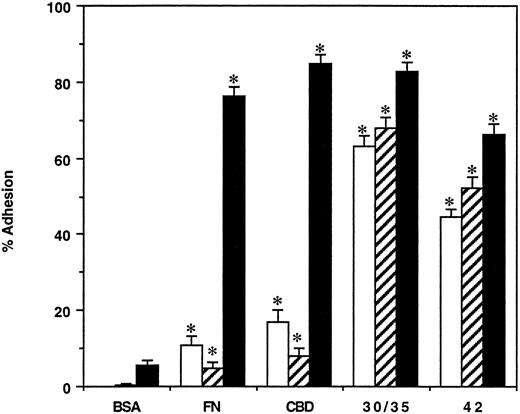
The results of these assays showed that the CBD and carboxy-terminal heparin-binding fragments were similar to intact FN in their ability to promote CFU-E attachment (Fig 2) using equimolar amounts of each polypeptide. Attachment of CFU-E was dependent on the amount of substrate applied to the wells; half-maximal attachment was observed at coating concentrations of 13 to 23 pmol/cm2 and maximal adhesion was observed at a concentration of 70 pmol/cm2 for all the substrates (data not shown). Low levels of attachment of mature and primitive BFU-E (9% and 20%, respectively) were observed to CBD, while much higher levels of adhesion were observed to the 42-kD (50%) and 30-/35-kD (65%) heparin-binding fragments (Fig 2). No increase in the adhesion of BFU-E to CBD was observed when the coating concentration was increased to 150 pmol/cm2 (data not shown). As observed previously,39 none of the erythroid progenitors bound to a 45-kD GBD fragment of FN (data not shown).
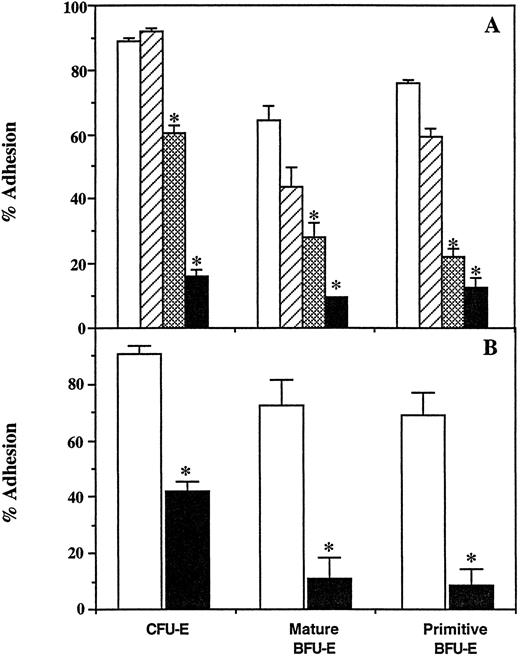
Adhesion of erythroid progenitors to FN and its chymotryptic fragments. Bone marrow cells were incubated in wells coated with BSA, FN, CBD, 30-/35-kD, and 42-kD fragments of FN at concentrations supporting maximal adhesion. After 2 hours, the unattached and attached populations were assayed for primitive BFU-E (□), mature BFU-E (▨), and CFU-E (▪). The results are expressed as the mean percent cell adhesion ± SEM (n ≥ 7), * P ≤ .02.
Adhesion of erythroid progenitors to FN and its chymotryptic fragments. Bone marrow cells were incubated in wells coated with BSA, FN, CBD, 30-/35-kD, and 42-kD fragments of FN at concentrations supporting maximal adhesion. After 2 hours, the unattached and attached populations were assayed for primitive BFU-E (□), mature BFU-E (▨), and CFU-E (▪). The results are expressed as the mean percent cell adhesion ± SEM (n ≥ 7), * P ≤ .02.
As mentioned, all attached BFU-E were assayed using the detachment method while CFU-E attached to the CBD and 42-kD fragments, like intact FN, were assayed by the overlay method. The total recovery of CFU-E and BFU-E using these methods was comparable (80% to 100%) to numbers obtained from wells coated with BSA. In contrast, only 20% to 40% of total CFU-Es were recovered from 30-/35-kD coated dishes when using the overlay method, although binding was still measured at approximately 70%. The colonies detected on the 30-/35-kD fragments were dispersed and individual erythroblasts were observed to be spread and enucleating. Because of this low recovery and altered morphology, all CFU-E binding to 30-/35-kD fragments in these studies was performed using the detachment method. This restored the colony morphology and increased the recovery from 30-/35-kD fragments to the levels obtained from BSA-coated dishes. The method used does not alter the results obtained because CFU-E binding to FN using the detachment method and CFU-E binding using the overlay method yielded identical results (data not shown).
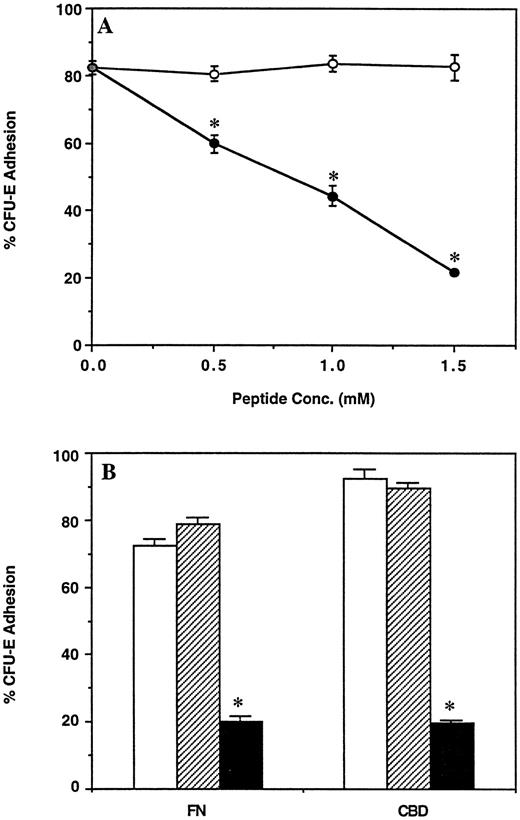
CFU-E adhesion to CBD is mediated by the RGDS sequence.Many cells, including MEL cells and human erythroid precursors, attach to the central CBD of FN through VLA-5 interaction with the RGDS sequence.25 39 In keeping with these findings, the synthetic hexapeptide GRGDSP inhibited CFU-E attachment to CBD in a dose-dependent fashion, whereas a control GRGESP peptide had no effect (Fig 3A). The inhibitory effect of the GRGDSP peptide was specific for CBD because this peptide did not affect adhesion of CFU-E to the 30-/35-kD heparin-binding fragments (data not shown).
(A) Effect of the synthetic peptide GRGDSP on adhesion of CFU-E to the CBD fragment of FN. Bone marrow cells were incubated in wells coated with CBD in the presence of the indicated concentrations of GRGDSP (•) or GRGESP (○) peptides. (B) Effect of integrin β1 subunit antibody on adhesion of CFU-E to FN and the CBD fragment of FN. Bone marrow cells were incubated in wells coated with FN or CBD in the absence of antibody (□), or in the presence of 100 μg/mL nonimmune rabbit IgG (▨), or 100 μg/mL β1 subunit antibody (▪). For both (A) and (B), the nonadherent and adherent populations of cells were then assayed for CFU-E and results are expressed as the mean percent cell adhesion ± SEM (n = 3), *P ≤ .02.
(A) Effect of the synthetic peptide GRGDSP on adhesion of CFU-E to the CBD fragment of FN. Bone marrow cells were incubated in wells coated with CBD in the presence of the indicated concentrations of GRGDSP (•) or GRGESP (○) peptides. (B) Effect of integrin β1 subunit antibody on adhesion of CFU-E to FN and the CBD fragment of FN. Bone marrow cells were incubated in wells coated with FN or CBD in the absence of antibody (□), or in the presence of 100 μg/mL nonimmune rabbit IgG (▨), or 100 μg/mL β1 subunit antibody (▪). For both (A) and (B), the nonadherent and adherent populations of cells were then assayed for CFU-E and results are expressed as the mean percent cell adhesion ± SEM (n = 3), *P ≤ .02.
To verify the role of β1 integrins in CFU-E adhesion to CBD, we performed antibody blocking experiments using IgG purified from a rabbit polyclonal antiserum which recognizes the β1 -subunit of the VLA-5 integrin.35 This antibody has previously been shown to block attachment of MEL cells and fibroblasts to FN and CBD. The presence of the β1 integrin on the CFU-E cell surface was verified when 60% of CFU-E bound to dishes coated with this β1 -subunit antibody (data not shown). When included in adhesion assays, the β1 -subunit antibody inhibited attachment of CFU-E to both FN and CBD (Fig 3B), while nonimmune rabbit IgG had no effect.
Erythroid progenitors bind to heparin-sensitive and heparin-resistant cell adhesion determinants on the carboxy-terminal heparin-binding fragments.As mentioned previously, both the 42-kD and 30-/35-kD fragments contain the carboxy-terminal HBD, while only the 30-/35-kD fragments contain at least part of the IIICS region. To determine the contribution of the HBD to erythroid progenitor adhesion, we measured the adhesion promoting activity of the 42- and 30-/35-kD fragments after pretreating these fragments with 50 mmol/L heparin. The results show that adhesion to the 42-kD fragment was almost completely abolished by pretreatment of this fragment with 50 mmol/L heparin (Table 2), suggesting that heparin completely blocked cellular interaction with the HBD. In contrast, heparin had no effect on the attachment of CFU-E to 30-/35-kD fragments, and only partially inhibited adhesion of mature and primitive BFU-E. Pretreatment of 30-/35-kD fragments with up to 100 mmol/L heparin yielded identical results (data not shown). These results show that adhesion of erythroid progenitors to the carboxy-terminal heparin binding fragments of FN involves recognition of both heparin-sensitive and heparin-resistant cell adhesion determinants. The heparin-resistant binding observed on 30-/35-kD fragments suggests that there are additional sites that promote primitive erythroid cell adhesion.
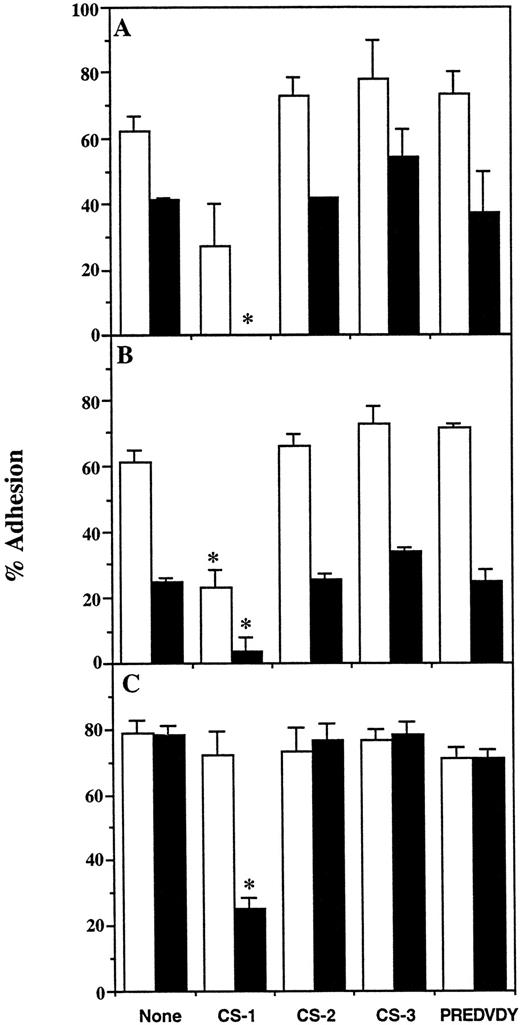
The CS-1 peptide inhibits binding of erythroid progenitors to the 30-/35-kD fragments.We synthesized three overlapping synthetic peptides, CS-1, CS-2, and CS-3, which together represent the first 68 amino acids of the IIICS region, and a heptapeptide PREDVDY (Fig 1B). Their effect on progenitor adhesion was examined by including the soluble peptides (1 mmol/L final concentration) in adhesion assays on heparin-treated and untreated 30/35 kD. This concentration was chosen based on studies showing maximal inhibition (>90%) of MEL cell binding to 30/35 kD by 1 mmol/L of CS-1 peptide (data not shown). Figure 4 shows that the CS-1 peptide significantly inhibited adhesion of primitive and mature BFU-E to untreated 30-/35-kD fragments and completely abolished binding on the heparin-treated fragments (Fig 4A and B). In contrast, 1 mmol/L CS-1 peptide had no effect on the adhesion of CFU-E to the untreated 30-/35-kD fragments, but significantly inhibited CFU-E attachment to the heparin-treated fragments (Fig 4C). Similar results were obtained when the concentration of CS-1 peptide was increased to 2 mmol/L in the adhesion assay (data not shown). Parallel incubations performed in the presence of CS-2, CS-3, and PREDVDY peptides had no significant effect on the adhesion of CFU-E and BFU-E, regardless of whether the 30-/35-kD preparation was pretreated with heparin. None of the peptides inhibited binding of erythroid progenitors to the 42-kD fragment (data not shown). The adhesion of both BFU-E and CFU-E to the 30-/35-kD heparin-binding fragments involves the recognition of heparin-dependent and -independent cell adhesion sites, and the latter includes the CS-1 sequence.
Effect of synthetic peptides on adhesion of erythroid progenitors to 30-/35-kD heparin binding fragments of FN. Bone marrow cells were incubated in wells coated with untreated (□) or heparin-treated (▪) 30-/35-kD fragments, in the absence or presence of 1 mmol/L CS-I, CS-2, CS-3, or PREDVDY synthetic peptides (see Fig 1B). The unattached and attached populations of cells were then assayed for primitive BFU-E (A), mature BFU-E (B), and CFU-E (C). Results are expressed as the mean percent cell adhesion ± SEM (n ≥ 2), * P ≤ .05.
Effect of synthetic peptides on adhesion of erythroid progenitors to 30-/35-kD heparin binding fragments of FN. Bone marrow cells were incubated in wells coated with untreated (□) or heparin-treated (▪) 30-/35-kD fragments, in the absence or presence of 1 mmol/L CS-I, CS-2, CS-3, or PREDVDY synthetic peptides (see Fig 1B). The unattached and attached populations of cells were then assayed for primitive BFU-E (A), mature BFU-E (B), and CFU-E (C). Results are expressed as the mean percent cell adhesion ± SEM (n ≥ 2), * P ≤ .05.
Adhesion of erythroid progenitors to the 30-/35-kD fragments involves the VLA-4 integrin.VLA-5 and VLA-4 integrins, which possess an identical β1 -subunit, have been shown to mediate the attachment of cells to the RGDS sequence and the CS-1 site of FN, respectively. A total of 60% to 80% of CFU-E and BFU-E bound to dishes coated with the β1 -subunit antibody, verifying the presence of the β1 integrin on their cell surfaces (data not shown). Adhesion of primitive and mature BFU-E to untreated 30-/35-kD fragments was dramatically inhibited in the presence of 100 μg/mL of β1 -subunit antibody (Fig 5A). This inhibition was further enhanced when cell attachment was measured on heparin-treated fragments (Fig 5A). The β1 -subunit antibody also inhibited adhesion of CFU-E to 30-/35-kD fragments, although this inhibition was most dramatic on heparin-treated fragments in the presence of 200 μg/mL antibody (Fig 5A). Attachment of CFU-E to the 42-kD fragment was not affected by the β1 -subunit antibody (data not shown). The same concentrations of nonimmune IgG had no effect on CFU-E or BFU-E attachment to either untreated or heparin-treated 30/35-kD fragments.
Effect of β1 subunit antibody and R1-2 monoclonal antibody on adhesion of erythroid progenitors to 30-/35-kD heparin binding fragments of FN. (A) Bone marrow cells were incubated in wells coated with untreated (□, ▧) or heparin-treated (▨, ▪) 30-/35-kD fragments in the absence (□, ▨) or presence (▧, ▪) of β1 subunit antibody. A total of 100 μg/mL of β1 subunit antibody was used for BFU-E assays, while 200 μg/mL was used for CFU-E assays. Unattached and attached population of cells were then assayed for erythroid progenitors. The level of adhesion in the presence of nonimmune IgG was identical to the adhesion observed in the absence of antibody. (B) Bone marrow cells were incubated in wells coated with 30/35-kD fragments in the absence (□) or presence (▪) of R1-2 hybridoma conditioned medium. A 0.5× concentration of R1-2 medium was used for BFU-E assays, while a 4× concentration was used for CFU-E assays. Unattached and attached populations of cells were then assayed for erythroid progenitors. Adhesion in the presence of concentrated control medium was identical to adhesion in either IMDM or in the presence of nonimmune IgG. The results are expressed as the mean percent cell adhesion ± SEM (n ≥ 2), * P ≤ .05.
Effect of β1 subunit antibody and R1-2 monoclonal antibody on adhesion of erythroid progenitors to 30-/35-kD heparin binding fragments of FN. (A) Bone marrow cells were incubated in wells coated with untreated (□, ▧) or heparin-treated (▨, ▪) 30-/35-kD fragments in the absence (□, ▨) or presence (▧, ▪) of β1 subunit antibody. A total of 100 μg/mL of β1 subunit antibody was used for BFU-E assays, while 200 μg/mL was used for CFU-E assays. Unattached and attached population of cells were then assayed for erythroid progenitors. The level of adhesion in the presence of nonimmune IgG was identical to the adhesion observed in the absence of antibody. (B) Bone marrow cells were incubated in wells coated with 30/35-kD fragments in the absence (□) or presence (▪) of R1-2 hybridoma conditioned medium. A 0.5× concentration of R1-2 medium was used for BFU-E assays, while a 4× concentration was used for CFU-E assays. Unattached and attached populations of cells were then assayed for erythroid progenitors. Adhesion in the presence of concentrated control medium was identical to adhesion in either IMDM or in the presence of nonimmune IgG. The results are expressed as the mean percent cell adhesion ± SEM (n ≥ 2), * P ≤ .05.
The monoclonal antibody R1-2 has previously been shown to specifically recognize the murine integrin α4 -subunit.37 A total of 70% to 95% of CFU-E and BFU-E bound to dishes coated with the R1-2 antibody, verifying the presence of the α4 integrin on their cell surfaces (data not shown). Figure 5B shows that a 0.5× concentration of hybridoma supernatant containing this antibody completely inhibited attachment of primitive and mature BFU-E to untreated 30-/35-kD fragments. Attachment of CFU-E was also sensitive to this antibody, although a 4× concentration of the hybridoma supernatant was required for 50% inhibition of CFU-E adhesion (Fig 5B). Similar results were obtained when CFU-E attachment was assayed on the heparin-treated 30-/35-kD fragments (data not shown). The R1-2 antibody had no effect on the RGDS-dependent adhesion of CFU-E to CBD or the attachment of CFU-E and BFU-E to the 42-kD fragment (data not shown). These results suggest that the CS-1–dependent adhesion of erythroid progenitors to the 30-/35-kD fragments is mediated by the VLA-4 integrin.
CS-1–containing fragments of FN promote maximal migration of CFU-E.Previous studies have shown that the binding sites of FN promote quantitatively and qualitatively distinct patterns of cellular migration. With the identification of differences in the adhesive properties of BFU-E and CFU-E and the suggestion that BFU-E are more migratory than CFU-E,20 it was of interest to examine the ability of each cell binding site to promote migration of erythroid progenitors. Bone marrow cells were incubated in the top well of blind well chambers containing either coated or uncoated filters as described. Migrated cells found in the bottom chamber and on the bottom of the filter were then assayed for erythroid progenitors. Because attempts to examine BFU-E migration using this method were unsuccessful, only results from CFU-E assays are presented here. The coating concentrations of substrates and the time of incubation were determined experimentally to promote maximal migration of CFU-E (data not shown). The results showed that the 30-/35-kD fragments promoted CFU-E migration at levels 11-fold over background, while migration on the CBD fragment was only threefold above background (Table 3). The level of migration on intact FN was approximately sixfold above control, half of that observed on the 30-/35-kD fragments. Migration on the 42-kD fragment was not significantly higher than background, suggesting that the ability of the 30-/35-kD fragments to promote high levels of migration may be dependent on cellular interaction with the CS-1 sequence. CFU-E did not migrate on the GBD fragment of FN or BSA, consistent with the inability of these substrates to promote adhesion of these cells. In addition, migration on the 30-/35-kD fragments does not appear to be chemotactic in nature, as soluble 30-/35-kD fragments in the bottom chamber did not support migration (data not shown). These results show that the cell binding regions of the FN molecule possess distinct adhesion- and migration-promoting activities for erythroid progenitors.
CFU-E Migration on FN and Fragments of FN
| Substrate . | No. . | Migration Index . | % Migration . |
|---|---|---|---|
| − | 29 | − | 2.5 ± 0.3 |
| FN | 26 | 5.7 ± 0.63-150 | 13.8 ± 1.93-150 |
| CBD | 21 | 3.1 ± 0.33-150 | 7.0 ± 1.03-150 |
| 30/35 kD | 22 | 11.3 ± 0.73-150 | 23.1 ± 2.53-150 |
| 42 kD | 12 | 1.7 ± 0.33-150 | 3.6 ± 0.53-150 |
| GBD | 3 | 1.2 ± 0.1 | 3.2 ± 0.3 |
| BSA | 3 | 0.6 ± 0.2 | 2.0 ± 0.8 |
| Substrate . | No. . | Migration Index . | % Migration . |
|---|---|---|---|
| − | 29 | − | 2.5 ± 0.3 |
| FN | 26 | 5.7 ± 0.63-150 | 13.8 ± 1.93-150 |
| CBD | 21 | 3.1 ± 0.33-150 | 7.0 ± 1.03-150 |
| 30/35 kD | 22 | 11.3 ± 0.73-150 | 23.1 ± 2.53-150 |
| 42 kD | 12 | 1.7 ± 0.33-150 | 3.6 ± 0.53-150 |
| GBD | 3 | 1.2 ± 0.1 | 3.2 ± 0.3 |
| BSA | 3 | 0.6 ± 0.2 | 2.0 ± 0.8 |
Bone marrow cells were incubated in blind well chambers containing filters coated with the indicated substrates. After 1 hour at 37°C, the bottom of the filters and bottom wells were assayed for the presence of CFU-Es. The results are presented as the mean migration index ± SEM and the mean percent migration ± SEM (n = number of experiments).
P ≤ .05.
DISCUSSION
Erythroid cell interactions with FN are thought to be critical for erythropoiesis, but the specific role(s) of each cell binding domain of FN in normal erythroid development is not known. The binding properties of erythroid cells during the later stages of erythropoiesis have been well characterized and are shown to be developmentally regulated. In these studies, we have characterized the binding of early normal murine erythroid progenitor cells to FN and found that adhesion to the RGDS, HBD, and CS-1 sequences of FN is also developmentally regulated during early erythroid differentiation. Because interaction with FN has been implicated in the migratory properties of cells, we also directly examined the ability of each cell binding domain of FN to promote migration of CFU-E. The results show that there are distinct erythroid cell adhesion and migration-promoting regions of FN and suggest that the regulation of erythroid cell interactions with FN during development may lead to changes in the migratory properties of these cells.
This study has shown that the ability of normal murine erythroid cells to bind to intact FN was acquired at the CFU-E stage of development (Table 1). Although BFU-E did not bind at high levels to intact FN, they were capable of binding to proteolytic fragments of FN. The CBD fragment, containing the RGDS sequence, promoted low (10% to 20%) but significant binding of BFU-E and 87% binding of CFU-E. These results differ from previous work showing that 60% of human BFU-E bound to RGDS-containing fragments of FN.28 This discrepancy may reflect differences in the adhesive properties between murine and human BFU-E. In contrast to CBD, 50% to 80% binding of both BFU-E and CFU-E was observed on the 30-/35-kD and 42-kD fragments. This binding involved interactions with both the HBD and the CS-1 sequence. The results of antibody competition assays (Figs 3 and 5) suggest that binding to the RGDS and CS-1 sequences was mediated by the previously characterized VLA-5 and VLA-4 integrins, respectively. Although no α5 antibody was available to confirm VLA-5 interaction with the RGDS sequence, the observation that β1 antibody blocked binding while the R1-2 antibody had no effect, together with the literature, suggests that VLA-5 is most likely mediating adhesion of CFU-E to the RGDS sequence.
The inability of BFU-E to bind significantly to intact FN in vitro, while binding to heparin-binding fragments of FN, may reflect conformational differences between purified FN and FN in association with other ECM components in vivo.40,41 Alternatively, the presence of proteolytic cleavage products of FN42 or other adhesive ligands for VLA-4 such as vascular cell adhesion molecule-143 may provide the necessary binding sites for BFU-E in vivo. Another possible explanation for the lack of BFU-E binding to FN may be the activation state of VLA-4 and/or VLA-5 on their cell surface. The activation state of these integrins has been shown to modulate the binding of cells to FN, and cytokines such as stem cell factor and IL-3 are known to activate both VLA-4 and VLA-5.44-49 It will be of interest to examine the effects of these growth factors on the adhesive properties of erythroid progenitors, particularly the ability of BFU-E to bind to intact FN.
The use of competitors, including heparin, synthetic peptides, and antibodies, in the adhesion assays has shown differences between BFU-E and CFU-E in their interactions with the 30-/35-kD fragments. For example, CFU-E adhesion to these fragments was completely resistant to the effects of heparin alone, while BFU-E binding was partially blocked (Fig 4). Similar results were obtained when the CS-1 peptide was used to inhibit binding. This partial inhibition of BFU-E binding may reflect heterogeneity within the BFU-E population itself. BFU-E were also found to be more sensitive to the effects of the anti-β1 and R1-2 antibodies than CFU-E (Fig 5). These results may again reflect a difference in the activation state of the VLA-4 integrin between CFU-E and BFU-E. At least two functional states of the VLA-4 integrin are known to exist and have been shown to influence hematopoietic cell interactions with FN.44
CFU-E adhesion to the 30-/35-kD fragments was completely resistant to the effects of CS-1 peptide in the absence of heparin pretreatment (Fig 4). In contrast, the R1-2 antibody, and to a lesser extent the anti-β1 antibody, partially inhibited CFU-E binding in the absence of heparin pretreatment (Fig 5). One possible explanation for these results is that these antibodies may be interfering with CFU-E binding to both the CS-1 sequence and the HBD, while the CS-1 peptide only interferes with interaction with the CS-1 sequence. Previous studies have shown that interactions with the FN-C/H I heparin-binding peptide within the HBD are mediated at least in part by the α4β1 integrin.29 Because neither the anti-β1 nor the R1-2 antibody affected binding of cells to the 42-kD fragment, specific interactions of VLA-4 with the FN-C/H I sequence do not appear to be critical for erythroid cell binding to the HBD in this case.
As mentioned earlier, adhesion to the CS-1 sequence and specific regions of the HBD involve contributions from both CSPGs and the VLA-4 integrin.13,14,29 Differences in the cell surface PG content between BFU-E and CFU-E could therefore have significant effects on cellular interactions with these sites. Expression of cell surface PGs are known to be developmentally regulated during hematopoiesis.50 Differences in the ability of BFU-E and CFU-E to bind to platelet factor 4 (PF4), a protein that binds glycosaminoglycan (GAG) side chains,51 suggest that cell surface PGs may also be developmentally regulated during erythropoiesis. We observed 80% of CFU-E and only 30% of BFU-E binding to PF4 (data not shown). This developmental change in PG content may also play a role in the differences observed between BFU-E and CFU-E in their binding to the 30-/35-kD fragments.
It has previously been suggested that erythroid progenitors migrate within the bone marrow from the bone surface toward the central sinus.31,32 This migration may be important for the formation of erythroblastic islands and the eventual release of reticulocytes into the general circulation.33 Based on the morphology of colonies produced in vitro, primitive BFU-E are thought to be more migratory than CFU-E.20 These studies are the first to directly measure the ability of erythroid progenitors to migrate on cell binding fragments of FN. We have shown that the CS-1–containing 30-/35-kD fragments supported the highest levels of CFU-E migration, twofold higher than intact FN, and fourfold higher than the CBD fragment. Migration on 30/35 kD was most likely due to interactions with the CS-1 sequence, as the 42-kD fragment, containing only the HBD, does not promote migration above background levels. Our results also suggest that interaction with the RGDS sequence, either in the CBD or intact FN, may result in decreased ability of CFU-E to migrate. These results are consistent with previous studies, which show that the CS-1 site promotes rapid random migration of neural crest cells, and that interaction with the RGDS sequence retards migration.52-54 These results also support the hypothesis that BFU-E are more migratory than CFU-E, as 90% of CFU-E interacted with the RGDS sequence, compared with only 10% to 20% of BFU-E.
The migration-promoting ability of the FN fragments agrees with the morphology of the CFU-E colonies produced when cells were grown in contact with each fragment (data not shown). While only half of the CFU-E colonies were recovered when cells were grown on the 30-/35-kD fragments, the colonies observed were very dispersed and the erythroblasts were spread and enucleating. In contrast, the morphology of CFU-E colonies on intact FN and CBD, which supported lower levels of migration than the 30-/35-kD fragments, was less dispersed and fewer erythroblasts were spread and enucleating. The CFU-E colonies grown on the 42-kD fragment, which did not support migration, were tightly packed and no enucleating or spread erythroblasts were detected.
This study has shown that the ability to promote both spreading and migration of erythroid cells resides mainly within the CS-1–containing region of FN. Because the CS-1 sequence is located in the alternatively spliced type IIICS region of FN, it is present in only one of three spliced variants in rat and two of five spliced variants in human.55 56 Thus, the presence or absence of CS-1, in addition to the expression of a functional VLA-4 integrin on the erythroid cell surface, may control the ability erythroid cells to migrate within the bone marrow. Together, our results suggest that erythroid cell binding to specific domains of FN is developmentally regulated during early murine erythroid differentiation, and these adhesive interactions have distinct functions that may influence the migratory properties of erythroid cells and play an important role in normal erythroid development.
Address reprint requests to Kristin L. Goltry, PhD, Aastrom Biosciences, 24 Frank Lloyd Wright Dr, Ann Arbor, MI 48105.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal