Abstract
CAAT/enhancer binding proteins (C/EBP) are a family of transcription factors that mediates adipocyte differentiation and the regulation of genes expressed in immune responses and inflammation, such as interleukin-6 (IL-6), IL-8, and granulocyte colony-stimulating factor (G-CSF ). We investigated the role of C/EBPβ (NF-IL6) in the generation of bone marrow B lymphocytes by taking advantage of C/EBPβ−/− mice. We found that the expansion of bone marrow (BM) B lymphocytes was impaired in long-term lymphoid cultures from C/EBPβ−/− mice. Consistent with this finding, the number of BM B cells was decreased in C/EBPβ−/− mice. Both the levels of IL-7 gene expression and bioactive IL-7 from BM stromal cells were decreased in C/EBPβ−/− mice. Furthermore, the proliferative responsiveness of BM B-cell precursors to IL-7 was also reduced as compared to wild-type mice, indicating that C/EBPβ is required for the generation of BM B cells induced by IL-7. Accordingly, IL-7 stimulates the C/EBPβ DNA-binding activity of normal BM pre-B lymphocytes as well as of 70Z/3 pre-B cells. These results point to C/EBPβ as a critical signaling molecule in BM B lymphopoiesis.
MEMBERS OF THE C/EBP1 family of transcription factors play important roles as regulators of cell proliferation and differentiation in response to extracellular stimuli.1-3 The best-characterized C/EBP protein is C/EBPβ (NF-IL6, IL-6DBP, CAP). C/EBPβ was first isolated and cloned as a transcription factor required for the induction of the IL-6 gene expression by interleukin-1 (IL-1).1 C/EBPβ can bind to the regulatory regions of various acute-phase protein genes and several cytokine genes, and transactivates the promoters of these genes.4-7 In normal tissues, C/EBPβ is expressed at undetectable or very low levels, but is rapidly induced by lipopolysaccharide (LPS) or by cytokines such as tumor necrosis factor-α (TNF-α) and IL-6.8 In addition, C/EBPβ expression increases during differentiation of myeloid and plasma cells,9-11 suggesting that C/EBPβ may also be involved in the differentiation of myeloid and lymphoid cell lineages. Other members of C/EBP family include C/EBPα, C/EBPδ, C/EBPγ, CHOP, and CRPI.12,13 Among them, C/EBPδ and C/EBPγ exhibit an expression pattern similar to that of C/EBPβ and has been shown to be involved in the regulation of several genes during inflammation and cell differentiation.7,14,15 To investigate specific immunoregulatory functions of C/EBPβ, we used C/EBPβ−/− mice16 to study the role of C/EBPβ in B-cell lymphopoiesis.
The development of B lymphocytes depends on ordered availability of cytokines acting on the proliferation and the differentiation of precursor cells.17 This implies that cytokine genes must be expressed upon the activation of specific transcription factors. However, little is known of the role of trans-activating factors in B-cell lymphopoiesis. We established Whitlock-Witte type long-term and short-term B-lymphoid cultures18 19 of C/EBPβ−/− mice bone marrow (BM) to investigate the role of C/EBPβ in B-cell lymphopoiesis. Our results showed an impaired generation of BM B lymphocytes in C/EBPβ−/− mice. We also found that BM stromal cells from C/EBPβ−/− mice expressed reduced levels of IL-7, and that B cells generated from short- as well as long-term cultures showed a reduced responsiveness to IL-7 in vitro. Accordingly, the DNA-binding activity of C/EBPβ was enhanced in nuclear extracts of both BM pre-B lymphocytes and 70Z/3 pre-B cells stimulated with IL-7, indicating that C/EBPβ plays a critical role in proper B-cell development.
MATERIALS AND METHODS
Reagents and animals.Purified recombinant mouse IL-7 was purchased from Pepro Tech Inc (Rocky Hill, NJ). Antimouse IL-7 monoclonal antibody (MoAb) was purchased from R&D systems Europe Ltd (Abingdon, UK). Polyclonal anti-C/EBPβ Ab was obtained from T. Kishimoto (Department of Medicine III, Osaka University, Osaka, Japan), anti-C/EBPδ(C-22) Ab was purchased from Santa Cruz Biotechnology Inc (Santa Cruz, CA), while a rabbit antiserum to C/EBPγ was provided by K. Calame (Department of Microbiology, Columbia University, New York, NY). Fluorescein isothiocyanate (FITC)-labeled antimouse IgM and PE antimouse CD45R/B220 were obtained from Pharmingen (San Diego, CA). Cell sorting experiments were performed as previously described20 by using the following reagents: CD45R/B220 (clone RA3-6B2) FITC-labeled,20 antimouse IgM clone LOmm-9 (Zymed, San Francisco, CA), PE-streptavidin (Molecular Probes, Eugene, OR). C/EBPβ−/− mice were generated as previously described.16 Procedures involving animals and their care were conducted in conformity with national and international laws and policies (EEC Council Directive 86/609, OJ L 358, December 12, 1987; Italian Legislative Decree 116/92, Gazzetta Ufficiale della Repubblica Italiana No. 40, February 18, 1992; NIH Guide for Care and Use of Laboratory Animals, NIH Publication 85-23, 1985).
Cell lines and lymphoid cell cultures.The CT6 cell line (a kind gift of Dr Brian M.J. Foxwell, The Kennedy Institute of Rheumatology, London, UK) was maintained in complete medium as described.21 The pre-B cell line 70Z/3 (a kind gift of N. Rice, Basic Research Program, NCI, FCRF, Frederick, MD) was maintained in RPMI 1640 supplemented with 10% heat-inactivated fetal calf serum (FCS) and 50 μmol/L 2-mercaptoethanol (2-ME). Long-term lymphoid cultures were performed as described18 with minor modifications. BM plugs from both 16- to 20-week-old C/EBPβ−/− and wild-type mice were suspended at 1 × 106/mL in tissue culture medium (RPMI 1640 medium/5% FCS /50 μmol/L 2-ME containing penicillin and streptomycin). Cells were cultured on 10-cm culture dishes and fed with fresh culture medium twice weekly. Nonadherent cells from cultures were examined weekly for B220-bearing cells by flow cytometry. For short-term cultures, CD45/B220+/IgM− or CD45/B220+/IgM− cells were isolated from BMs of C/EBPβ null or C/EBPβ wild-type mice by FACS sorting, and cultured in 96-well flat-bottom plates at 2 × 104/0.1 mL culture medium containing RPMI 1640 medium, 15% FCS, 50 μmol/L 2-ME, minimum essential medium (MEM), vitamin, and different concentrations of recombinant murine IL-7 (0.01 to 100 ng/mL), as previously reported.19 The cultures were fed at day 4 with equal volume of fresh medium containing IL-7. On day 7, the cultures were pulsed with 1 μCi/well of [3H]-thymidine (Amersham, Milan, Italy) for 6 hours and harvested. The incorporated radioactivity were determined in a liquid scintillation counter. Isolation of BM adherent cells was obtained as described.22 Briefly, after 2 weeks of culture of BM cells (1 × 107/dish) a confluent adherent layer of cells was established. All culture medium was removed from the cultures and replaced with fresh medium containing 5 μg/mL of mycophenolic acid (MPA) (Sigma Chemicals, St Louis, MO). Three days later, all the MPA-containing medium was replaced with fresh medium. After an additional 3-day incubation, the cycle was repeated. Cultures were maintained for at least 1 week following the last MAP treatment before use. Cell-surface analysis of IL-7 receptors was performed by using a biotinylated IL-7 following streptavidin-PE (R & D Systems Europe Ltd), according to the manufacturer's specifications. At different culture times, nonadherent cells were collected from long-term cultures of BM, washed twice with RDF1 buffer, and resuspended at a final concentration of 4 × 106 cells/mL. Ten microliters of biotinylated IL-7 (10 μg/mL) was added to 25 μL of cell suspensions. Cells were incubated for 1 hour at 4°C, washed, incubated with streptavidin-PE together with FITC-CD45/B220 MoAb, and analyzed by flow cytometry. The isolation of highly purified B220+/IgM− or B220+/IgM+ BM cells was achieved by a Becton Dickinson FACStar Plus (Milan, Italy) equipped with argon and argon-dye lasers as described.20
IL-7 bioassay.IL-7 bioactivity in culture supernatant from MPA-treated BM adherent cells was determined by adding serial twofold dilutions of the test supernatants or of an IL-7 standard preparation to 5 × 104 CT6 cells in 100 μL/well in triplicate cultures. After 18 hours of incubation at 37°C, cells were pulsed with 1 μCi [3H]-thymidine for the final 6 hours before harvesting. In neutralizing assays, cell culture supernatants or a standard preparation of IL-7 was preincubated with anti–IL-7 antibody or with an isotype-matched mouse IgG (Sigma Chemicals) for 30 minutes at 37°C as reported.19
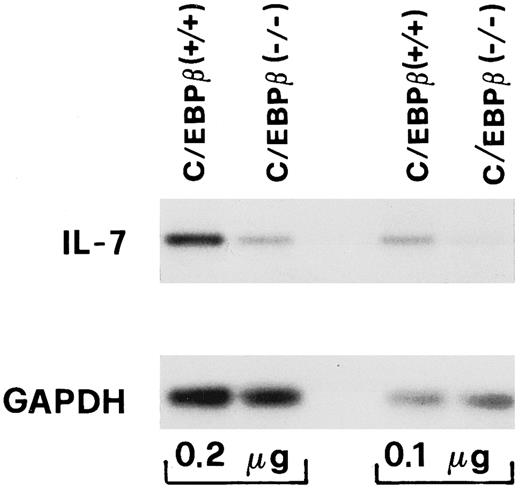
RNA extraction and IL-7 reverse transcriptase-polymerase chain reaction (RT-PCR).Total RNA was prepared from MPA-treated adherent cells by cell lysis in guanidine-thiocyanate buffer and acid phenol/chloroform extraction, followed by ethanol precipitation.23 Reverse transcription of mRNA and PCR amplification of cDNA were performed as previously described.24 Briefly, RNA was incubated with buffer, dNTP, random hexamers, RNAse inhibitor, and MuLV-reverse transcriptase at 42°C for 30 minutes. PCR amplification of cDNAs were performed using the following condition: 94°C for 1 minute, 57°C for 1 minute, and 72°C for 2 minutes for 20 cycles. The cycle number was chosen to generate a linear dose-response curve and the amounts of cDNA to be amplified was normalized using the relative expression level of GAPDH. Murine (m) IL-7 and hGAPDH primers were synthesized based on the published cDNA sequence25,26: IL-7 (sense): 5′-ACATCATCTGAGTGCCACA-3′; IL-7 (antisense): 5′-CTCTCAGTAGTCTCTTTAG-3′; GAPDH (sense) 5′-CCATGGAGAAGGCTGGGG-3′; GAPDH (antisense) 5′-CAAAGTTGTCATGGATGACC-3′. The expected amplified PCR products are 355 bp and 194 bp in the case of mIL-7 and mGAPDH, respectively. After amplification, the PCR products were analyzed by agarose gel electrophoresis and Southern blot hybridization with [32P]-radiolabeled mIL-7 cDNA and human GAPDH cDNA probes. Densitometric analysis of IL-7 and GAPDH expression was performed by using an LKB 2202 Ultrascan Laser densitometer (Farmacia, Milan, Italy) assisted by a Hewlett-Packard 3390A integrator (Milan, Italy).
Electrophoretic mobility shift assay.BM pre-B lymphocytes and 70Z/3 pre-B cells were isolated and cultured as described above. BM pre-B lymphocytes were cultured for 16 hours without IL-7 and stimulated with 10 ng of IL-7 for 2 hours in parallel with 70Z/3 pre-B cells. Nuclear extracts and gel-shift assays were performed as described elsewhere.27 28 After incubation, cells were obtained, washed once in cold phosphate-buffered saline (PBS), and transferred to 1.7-mL microfuge tubes for a second wash in cold PBS. The supernatant was removed and the cell pellet was resuspended in lysing buffer (10 mmol/L HEPES pH 7.9, 1 mmol/L EDTA, 60 mmol/L KCl, 1 mmol/L dithiothreitol [DTT], 1 mmol/L phenylmethylsulfonyl fluoride [PMSF], 0.2% V/V Nonidet P-40 [Sigma Chemicals, Milan, Italy]) for 5 minutes. Nuclei were collected by centrifugation (500g, 5 minutes), rinsed with NP-40-free lysing buffer, and resuspended in 150 μL of buffer containing 250 mmol/L Tris HCl pH 7.8, 20% glycerol, 0.42 mol/L NaCl, 60 mmol/L KCl, 1 mmol/L DTT, and 1 mmol/L PMSF. Nuclei were then subjected to three cycles of freezing and thawing. The suspension was cleared by centrifugation (7,000g, 15 minutes), and aliquots were immediately tested in gel retardation assay or stored in liquid phase N2 until use. Double-stranded oligonucleotides spanning the C/EBPβ site of the IL6 gene (5′-GATCGGACGGTCACATTGCACAATCTTAATTAAT-3′) were synthesized, purified, annealed, and end-labeled with [γ-32]-ATP (Amersham Corp, Arlington Heights, IL) using polynucleotide kinase (New England Biolabs, Beverly, MA). Equal amounts of cell extracts (5 μg) were incubated in a reaction mixture consisting of 20 μL buffer containing 20 mmol/L HEPES, pH 7.9, 20% glycerol, 100 mmol/L KCl, 0.2 mmol/L EDTA, 0.5 mmol/L DTT, 0.5 mmol/L PMSF, 2 μg poly [d(I-C)] (Boehringer Mannheim, Milan, Italy), for 5 minutes on ice. One microliter of [γ-32P]-labeled double-stranded probe (0.2 ng, 4 to 6 × 104 cpm) was then added with or without a 100-fold molar excess of wild-type competitor oligonucleotide. In some samples, 1 μL of polyclonal Ab to C/EBPβ, C/EBPδ, or to C/EBPγ was added to the reaction mixtures. The reactions were incubated at room temperature for 30 minutes and run on a 5% acrylamide/bis-acrylamide (30:1) gel in 22.5 mmol/L Tris-borate, 0.5 mmol/L EDTA. Gels were dried and autoradiographed.
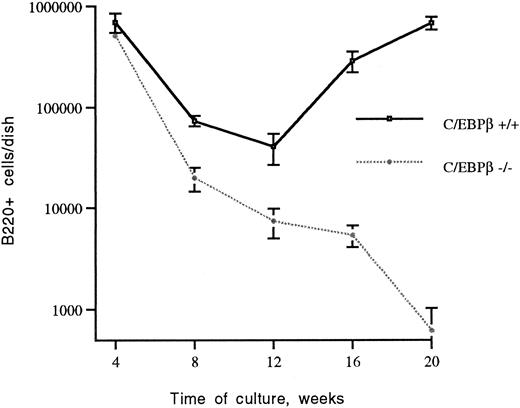
Generation of B lymphocytes in long-term cultures of BMs of C/EBPβ+/+ and C/EBPβ−/− mice. BM cells of C/EBPβ+/+ mice and C/EBPβ−/− mice were cultured at 106/mL in 10-cm tissue-culture dishes as detailed in Materials and Methods. At the indicated times, cultures were examined by flow cytometry for the number of B220+ cells. The data are expressed as the mean ± the standard deviations of five independent experiments.
Generation of B lymphocytes in long-term cultures of BMs of C/EBPβ+/+ and C/EBPβ−/− mice. BM cells of C/EBPβ+/+ mice and C/EBPβ−/− mice were cultured at 106/mL in 10-cm tissue-culture dishes as detailed in Materials and Methods. At the indicated times, cultures were examined by flow cytometry for the number of B220+ cells. The data are expressed as the mean ± the standard deviations of five independent experiments.
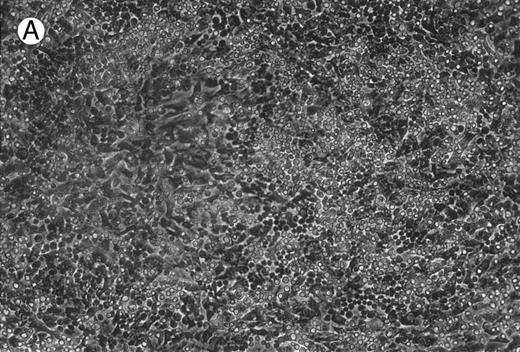
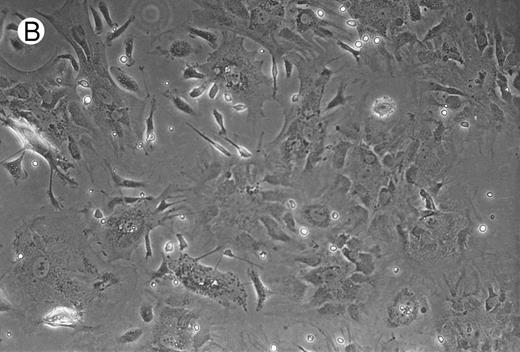
Micrographs of long-term cultures of B lymphocytes. Micrographs of BM of C/EBPβ+/+ mice (A) and C/EBPβ−/− mice (B) (original magnification × 430). Pictures were taken at 14-week cultures.
Micrographs of long-term cultures of B lymphocytes. Micrographs of BM of C/EBPβ+/+ mice (A) and C/EBPβ−/− mice (B) (original magnification × 430). Pictures were taken at 14-week cultures.
RESULTS AND DISCUSSION
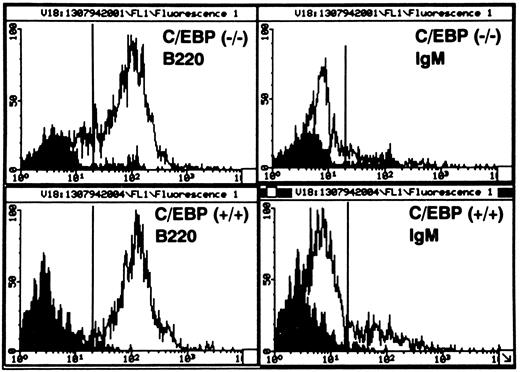
The generation of B lymphocytes is impaired in long-term cultures from BM of C/EBPβ−/− mice.We examined the effects of the C/EBPβ null mutation on long-term B-lymphoid proliferation using CD45/B220 as a general marker of B cells, and the presence of surface IgM as indicative of mature B cells.29 30 In the early phase of the BM culture (up to 4 weeks), B220-bearing cells from C/EBPβ−/− mice did not show any significant difference compared with equivalent cells from cultures of wild-type mice. Starting at 12 weeks, the generation of B220-bearing cells from C/EBPβ−/− mice was significantly decreased, leaving few nonadherent cells after 16 weeks (Fig 1). In marked contrast, the number of B220+ cells from wild-type mice increased after 12 weeks of culture to about 1 × 106 per dish at 20 weeks (Fig 1). Although the generation of B lymphocytes in long-term cultures was decreased in C/EBPβ−/− mice (shown in Fig 2), the distribution of B220+/IgM− (pre-B cells), and B220+/IgM+ (mature B cells) populations among the nonadherent cells did not show any significant difference between C/EBPβ−/− and wild-type mice. In fact, greater than 90% of cells were B220+ B cells, with 78% carrying the B220+/IgM− phenotype (pre-B cells), and 22% expressing the B220+/IgM+ phenotype (mature B cells) (Fig 3). This indicates that C/EBPβ deficiency affects selectively the expansion of pre-B lymphocytes from BM precursors, but it does not interfere with B-cell differentiation.
Flow cytometric analysis of nonadherent cells generated in long-term cultures of BM stromal cells. Cells were doubly stained with PE-conjugated antimouse CD45/B220 and with FITC-conjugated antimouse IgM. Greater than 90% of cells are B220+ B cells, with 78% carrying the B220+/IgM− phenotype (pre-B cells), and 22% expressing the B220+/IgM+ phenotype (mature B cells). Solid peaks indicate the staining with isotype-matched MoAb controls. The data are representative of the weekly analysis of BM cultures from 10 individual C/EBPβ+/+ and C/EBPβ−/− mice.
Flow cytometric analysis of nonadherent cells generated in long-term cultures of BM stromal cells. Cells were doubly stained with PE-conjugated antimouse CD45/B220 and with FITC-conjugated antimouse IgM. Greater than 90% of cells are B220+ B cells, with 78% carrying the B220+/IgM− phenotype (pre-B cells), and 22% expressing the B220+/IgM+ phenotype (mature B cells). Solid peaks indicate the staining with isotype-matched MoAb controls. The data are representative of the weekly analysis of BM cultures from 10 individual C/EBPβ+/+ and C/EBPβ−/− mice.
IL-7 gene expression of BM stromal cells is decreased in C/EBPβ−/− mice.Generation of precursor B cells in BM depends on stromal cells that act through intracellular contact and via the secretion of growth factors.31 These include IL-7, a cytokine that promotes survival and expansion of early B cells in BM stromal cultures and in vivo.32-34 To determine whether the defective B-lymphoid proliferation in BM of C/EBPβ−/− mice was related to a decrease in the availability of cytokines involved in B-cell development, we measured IL-7 gene expression from BM stromal cells and the levels of IL-7 in supernatants of long-term cultures of BM stromal cells. Total RNA was prepared from MPA-treated BM adherent cells and the mRNA levels of IL-7 were evaluated by RT-PCR. As shown in Fig 4, IL-7 mRNA expression was about fourfold lower in the C/EBPβ−/− mice than in the wild-type mice, as seen by densitometric analysis (not shown). The decreased IL-7 mRNA expression was consistent with the lower level of bioactive IL-7 in the culture supernatants of MPA-treated BM adherent cells (Table 1). In these experiments, addition of a neutralizing antibody to IL-7 resulted in about a 75% decrease in the CT-6 proliferative response, suggesting that other factors could participate in the generation of B cells from long-term cultures of BM (Table 1). The levels of other cytokines such as IL-3, IL-4, IL-6, IL-8, IL-10, granulocyte colony-stimulating factor (G-CSF ), and TNF-α in culture supernatants of BM adherent cells from C/EBPβ−/− and wild-type mice were substantially unchanged (data not shown). These results indicate that deletion of C/EBPβ leads to a selective decrease in IL-7 production from stromal BM cells, and could be partly responsible for the impaired development of B lymphocytes of C/EBPβ−/− mice.
IL-7 mRNA expression from BM adherent cells of C/EBPβ+/+ and C/EBPβ−/− mice. Total RNA was extracted from MPA-treated BM adherent cells of C/EBPβ+/+ and C/EBPβ−/− mice. RT-PCR with mouse IL-7–specific primers was performed from the indicated amounts of RNA (0.1 μg, 0.2 μg). IL-7 mRNA expression was visualized by a Southern blot analysis of RT-PCR products in parallel with constitutively expressed GAPDH by using a mouse IL-7 or a human GAPDH cDNA.
IL-7 mRNA expression from BM adherent cells of C/EBPβ+/+ and C/EBPβ−/− mice. Total RNA was extracted from MPA-treated BM adherent cells of C/EBPβ+/+ and C/EBPβ−/− mice. RT-PCR with mouse IL-7–specific primers was performed from the indicated amounts of RNA (0.1 μg, 0.2 μg). IL-7 mRNA expression was visualized by a Southern blot analysis of RT-PCR products in parallel with constitutively expressed GAPDH by using a mouse IL-7 or a human GAPDH cDNA.
Levels of Bioactive IL-7 in Culture Supernatants of BM Adherent Cells From C/EBPβ(+/+) and C/EBPβ(−/−) Mice
| . | IL-7 Production (ng/mL)* . | |
|---|---|---|
| . | Mice† . | . |
| . | . | . |
| . | C/EBPβ+/+ . | C/EBPβ−/− . |
| Exp 1 | 4.5 | 1.1†‡ |
| Exp 2 | 4.2 | 1.8 |
| Exp 3 | 5.1 | 2.1 |
| Exp 4 | 3.8 | 1.2 |
| Exp 5 | 4.5 | 2.0 |
| Exp 6 | 4.0 | 1.3 |
| Meanρ | 4.417 | 1.583 |
| Standard deviation | 0.426 | 0.436 |
| Significance | 0.001 | |
| . | IL-7 Production (ng/mL)* . | |
|---|---|---|
| . | Mice† . | . |
| . | . | . |
| . | C/EBPβ+/+ . | C/EBPβ−/− . |
| Exp 1 | 4.5 | 1.1†‡ |
| Exp 2 | 4.2 | 1.8 |
| Exp 3 | 5.1 | 2.1 |
| Exp 4 | 3.8 | 1.2 |
| Exp 5 | 4.5 | 2.0 |
| Exp 6 | 4.0 | 1.3 |
| Meanρ | 4.417 | 1.583 |
| Standard deviation | 0.426 | 0.436 |
| Significance | 0.001 | |
Supernatants collected 24 hours postfeeding were tested for IL-7 bioactivity by using the CT-6 cells.21 In parallel experiments, linear dilutions of neutralizing MoAb to IL-7 were added to the cultures. The data were compared with a standard curve of recombinant IL-7 (0.01 to 100 ng/mL).
Adherent cells were isolated from MPA-treated BM of C/EBPβ null mice and of wild-type littermates as detailed in Materials and Methods.
The data are corrected according to the inhibition observed in the cultures supplemented with the anti–IL-7 MoAb, and represent the actual levels of IL-7 production.
ρ The analysis of the data was performed according to the paired Student's t-statistic by using a StatWorks program (Cricket Software Inc, Philadelphia, PA).
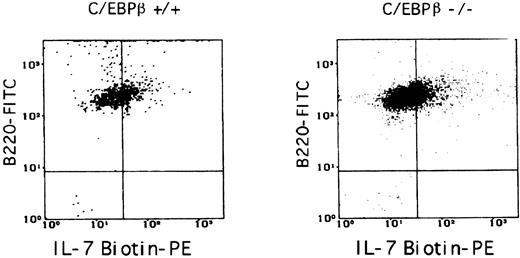
IL-7 responsiveness of B-cell precursors from BM of C/EBPβ−/− mice is impaired.C/EBPβ mediates the cell growth and differentiation induced by certain cytokines, including IL-6, IL-8, and G-CSF.1,9 35 To test whether the C/EBPβ null mutation might result in a decreased responsiveness of C/EBPβ−/− pre-B cells to IL-7, highly purified B220+/IgM− or B220+/IgM+ B cells were isolated from BMs of C/EBPβ−/− mice and their wild-type littermates by FACS sorting (Fig 5A). The cells were then analyzed for their ability to proliferate upon IL-7 stimulation. We observed that the proliferative response of BM B-cell precursors from C/EBPβ−/− mice was dramatically reduced as measured by [3H]-thymidine incorporation and by viable cell counts (Fig 5B). In contrast, no difference was observed in the proliferative response of spleen B lymphocytes to IL-4 in C/EBPβ−/− and wild-type mice, indicating the absence of an intrinsic proliferative defect in B cells from C/EBPβ−/− mice (data not shown). The flow cytometric analysis of IL-7 receptors on B220+ B cells either derived from long-term cultures or from short-term cultures of BM showed equivalent levels of IL-7 receptors both in C/EBPβ−/− and wild-type mice, indicating that the observed lack of IL-7 responsiveness was not caused by decreased levels of IL-7 receptors (Fig 6).
Proliferative response of BM B-lymphocyte subpopulations of C/EBPβ+/+ and C/EBPβ−/− mice to IL-7 stimulation. (A) FACS analysis of sorted B-cell subpopulations. BM cells from C/EBPβ+/+ and C/EBPβ−/− mice were stained with CD45/B220-FITC and with antimouse IgM-biotin followed by streptavidin-PE labeling. Cells were sorted in CD45/B220+/IgM− and CD45/B220+/IgM+ cell subsets by FACS sorting, as detailed in Materials and Methods. A purity of greater than 99.5% was consistently achieved. (B) Response of BM B lymphocytes from C/EBPβ+/+ or C/EBPβ −/− mice to IL-7 stimulation. CD45/B220+/IgM− and CD45/B220+/IgM+ cell subsets were isolated from BM cells of C/EBPβ+/+ and C/EBPβ −/− mice, cultured into 96-well plates at 2 × 104/0.1 mL, and stimulated with the indicated amounts of IL-7. On day 7, cultures were obtained to determine [3H]-thymidine incorporation. The data are expressed as the mean ± the standard deviations of three independent experiments. Similar results were obtained in the case of several independent experiments with B-cell populations obtained from long-term B-cell cultures (not shown).
Proliferative response of BM B-lymphocyte subpopulations of C/EBPβ+/+ and C/EBPβ−/− mice to IL-7 stimulation. (A) FACS analysis of sorted B-cell subpopulations. BM cells from C/EBPβ+/+ and C/EBPβ−/− mice were stained with CD45/B220-FITC and with antimouse IgM-biotin followed by streptavidin-PE labeling. Cells were sorted in CD45/B220+/IgM− and CD45/B220+/IgM+ cell subsets by FACS sorting, as detailed in Materials and Methods. A purity of greater than 99.5% was consistently achieved. (B) Response of BM B lymphocytes from C/EBPβ+/+ or C/EBPβ −/− mice to IL-7 stimulation. CD45/B220+/IgM− and CD45/B220+/IgM+ cell subsets were isolated from BM cells of C/EBPβ+/+ and C/EBPβ −/− mice, cultured into 96-well plates at 2 × 104/0.1 mL, and stimulated with the indicated amounts of IL-7. On day 7, cultures were obtained to determine [3H]-thymidine incorporation. The data are expressed as the mean ± the standard deviations of three independent experiments. Similar results were obtained in the case of several independent experiments with B-cell populations obtained from long-term B-cell cultures (not shown).
Surface expression of IL-7 receptors on B cells from C/EBPβ+/+ or C/EBPβ−/− mice. B lymphocytes obtained from long-term cultures of BM stromal cells were stained with B220-FITC MoAb and with IL-7 biotin, followed by incubation with streptavidin-PE. 31.5% and 34.7% of cells expressed IL-7 receptors in the case of C/EBPβ+/+ or C/EBPβ−/− mice, respectively. Similar results were obtained in the case of BM B-cell subpopulations (not shown).
Surface expression of IL-7 receptors on B cells from C/EBPβ+/+ or C/EBPβ−/− mice. B lymphocytes obtained from long-term cultures of BM stromal cells were stained with B220-FITC MoAb and with IL-7 biotin, followed by incubation with streptavidin-PE. 31.5% and 34.7% of cells expressed IL-7 receptors in the case of C/EBPβ+/+ or C/EBPβ−/− mice, respectively. Similar results were obtained in the case of BM B-cell subpopulations (not shown).
IL-7 can induce C/EBPβ DNA-binding activity in pre-B cells.Results shown in Fig 5B suggested that C/EBPβ transcription factors could mediate the responsiveness of pre-B cells to IL-7. To test whether C/EBP factors were indeed activated in response to IL-7, nuclear extracts were prepared from IL-7–stimulated B220+/IgM− B lymphocytes isolated by fluorescence-activated cell sorting (FACS) sorting from BM cells of C/EBPβ wild-type mice, as well as 70Z/3 pre-B cells, and tested for binding to a oligonucleotide corresponding to the C/EBP binding site of IL-6 promoter.6 We found that IL-7 stimulation induced a strong increase in C/EBP DNA-binding activity in both BM pre-B cells and in 70Z/3 pre-B cell line (Fig 7). In these experiments addition of antibodies to C/EBPβ, C/EBPδ, or to C/EBPγ resulted in a decrease in DNA-binding, indicating the presence of C/EBPβ, C/EBPδ, and C/EBPγ isoforms in the protein-DNA complexes. In the case of 70Z/3 pre-B cells, no inhibition of C/EBP DNA-binding activity was observed in nuclear extracts tested in the presence of the anti-C/EBPδ antiserum, indicating that IL-7 does not induce the C/EBPδ isoform in this cell line (Fig 7). These results identify C/EBP proteins as an intracellular signaling molecule of IL-7.
Induction of C/EBP binding activities by IL-7. BM B220+/IgM− pre-B cells were isolated by FACS sorting as detailed in Materials and Methods. BM pre-B lymphocytes and 70Z/3 pre-B cells were stimulated with IL-7 (10 ng/mL) for 2 hours or left untreated. Nuclear extracts were prepared and incubated with [32P]-labeled C/EBP double-strand oligonucleotide, as described in Materials and Methods. Unlabeled competitor probe was used at 100-fold molar excess. One milliliter of the indicated anti-C/EBP antisera or of preimmune rabbit serum was added to the reaction mixtures. The results of a single experiment are shown as a representative of four other independent experiments.
Induction of C/EBP binding activities by IL-7. BM B220+/IgM− pre-B cells were isolated by FACS sorting as detailed in Materials and Methods. BM pre-B lymphocytes and 70Z/3 pre-B cells were stimulated with IL-7 (10 ng/mL) for 2 hours or left untreated. Nuclear extracts were prepared and incubated with [32P]-labeled C/EBP double-strand oligonucleotide, as described in Materials and Methods. Unlabeled competitor probe was used at 100-fold molar excess. One milliliter of the indicated anti-C/EBP antisera or of preimmune rabbit serum was added to the reaction mixtures. The results of a single experiment are shown as a representative of four other independent experiments.
In this report we focused on the proliferation of B lymphocytes from BM, which is the primary organ for B lymphopoiesis in adult mice. Our results show that C/EBPβ is required for a proper generation of BM lymphocytes. We found that this deficiency occurred at the pre-B cell stage and was dependent on IL-7, as suggested by the decreased IL-7 production from C/EBPβ−/− stromal cells and by the low response of BM B-cell precursors derived from C/EBPβ−/− mice to IL-7. The defective responsiveness of BM pre-B cells to IL-7 is likely to play a major role in the impaired development of B-cell precursors. In fact, no significant improvement was observed when exogenous IL-7 was added in short-term cultures of BM, or when supernatants from BM cultures of wild-type mice were added to BM cultures of C/EBPβ−/− mice (data not shown). As BM B lymphocytes from C/EBPβ−/− mice or from their wild-type littermates expressed equivalent levels of IL-7 receptors (Fig 6), the above data indicate that C/EBPβ plays a major role in the generation of BM pre-B cells in response to IL-7.
After 25 to 30 weeks of age, the C/EBPβ−/− mice showed enhanced lymphoid proliferation in vivo, such as splenomegaly.16 In that early study, the BM compartments of C/EBPβ−/− mice were not examined.16 This prompted us to determine the levels of B220+/IgM− or B220+/IgM+ B cells in BMs of C/EBPβ−/− or wild-type mice. As shown in Table 2, both the percentages and the absolute numbers of mature B cells as well as B220+/IgM− precursor B cells were reduced in C/EBPβ−/− BMs. This apparent discrepancy may be explained by the fact that different phases of B-cell development take place in specific lymphoid organs. B-lymphocyte differentiation can be distinguished into two phases: an antigen-independent step which occurs in the BM (the primary lymphoid organ) and the antigen-dependent phase which takes place in the secondary lymphoid organ (eg, spleen lymph nodes, tonsils).36 In the BM, the antigen-independent phase of B-cell differentiation is heavily dependent on cytokines derived from stromal cells, such as IL-7. Differently, the antigen-dependent phase of B-cell differentiation in the secondary organs is mostly dependent on cytokines derived from T lymphocytes such as IL-2, IL-4, IL-6, and interferon-γ.7,37-39 Moreover, B-cell responses likely result from the combined effects of several cytokines.40-42 Indeed, the peripheral lymphoproliferation observed in the C/EBPβ−/− mice was age-dependent, and is likely to be of reactive origin due to a disregulation of the T-helper–dependent antibody response.16 In addition, a compensatory mechanism occurring in vivo may be in part responsible for the expansion of the peripheral B-cell compartment in C/EBPβ−/− mice. It is noteworthy that myelofibrosis syndromes are associated with splenomegaly and extramedullary hemopoiesis.43
Phenotypic Analysis of B Lymphocytes From BM of C/EBPβ+/+ and C/EBPβ −/− Mice
| . | Mice* . | |
|---|---|---|
| . | C/EBPβ+/+ . | C/EBPβ−/− . |
| Total cell yield | 49.2 × 106 | 41.1 × 106 |
| (4.9) | (6.8) | |
| B220+ cells† | 14.2 | 6.1 |
| (1.6) | (0.7) | |
| B220+/IgM−† | 8.8 | 3.9 |
| (1.0) | (0.4) | |
| B220+ cell yield‡ | 7.0 × 106 | 2.4 × 106 |
| (0.9) | (0.3) | |
| . | Mice* . | |
|---|---|---|
| . | C/EBPβ+/+ . | C/EBPβ−/− . |
| Total cell yield | 49.2 × 106 | 41.1 × 106 |
| (4.9) | (6.8) | |
| B220+ cells† | 14.2 | 6.1 |
| (1.6) | (0.7) | |
| B220+/IgM−† | 8.8 | 3.9 |
| (1.0) | (0.4) | |
| B220+ cell yield‡ | 7.0 × 106 | 2.4 × 106 |
| (0.9) | (0.3) | |
The data are the mean of 26 C/EBPβ null mice and of an equal number of wild-type littermates. The relative standard deviations are shown in parentheses.
Single cell suspensions of cells were prepared from BM and analyzed by double fluorescence as detailed in Materials and Methods.
Values are expressed as percentages of B220+ or B220+/IgM− cells detected in the total BM cell population.
B220+ cell yield was calculated according to the percentages of B220+ BM cells.
C/EBPβ binding motifs have been identified in the functional regulatory regions of several genes such as G-CSF, TNF-α, IL-1, IL-6, and IL-8.2,6,9,35,44 We show here that both the IL-7 production from stromal cells and the proliferative response of pre-B lymphocytes to IL-7 are impaired in C/EBPβ−/− mice. This suggests that C/EBPβ is involved both in the regulation of the IL-7 gene expression, and in mediating the IL-7 effects on B cells, particularly those related to the proliferative response of B-cell precursors to IL-7. In this regard, C/EBPβ could cooperate with other proteins, such as STAT1, STAT3, and STAT5, which function as tyrosine phosphorylated transcription factors in mediating the IL-7 signal transduction.45,46 The molecular basis of the role of C/EBPβ in B-lymphoid proliferation is not clear. The proliferation of IL-7–dependent B-cell precursors from BM also requires cell contacts involving CD44 and Hyaluronate, interaction of c-kit with stem cell factor (SCF ) and IL-7 receptors.47,48 Moreover, other factors such as insulin-like growth factor 1 (IGF-1), pre–B-cell growth stimulatory factor (pBSF ), pre–B-cell costimulatory factor (BST-1), and Pim-1 gene product were reported to potentiate the B-cell proliferative response.49-52 This suggests that C/EBPβ deficiency may lead to an impaired generation of B-cell precursors by acting at multiple pathways of B-cell stimulation.
As shown in Fig 7, we observed that IL-7 is a strong inducer of the DNA-binding activity of C/EBP. Despite the similarity in the pattern of expression between C/EBPβ and C/EBPδ in normal tissues, the activities of C/EBPβ and C/EBPδ are induced by different mechanisms: posttranslational for C/EBPβ and transcriptional for C/EBPδ.3 In fact, activation of C/EBPβ requires threonine phosphorylation induced through the ras-raf-MAP kinases.53 This implies the possibility that IL-7 could activate the ras pathway of signal transduction, in addition to the documented activation of STAT proteins through the γ-chain of the IL-7 receptor.54 An impaired B-lymphoid development was observed in IL-7−/− and IL-7R−/− mice.55,56 The former showed the BM B lymphopoieis blocked at the transition from pro-B to pre-B cells,55 and the latter displayed a reduction in IL-7 responsiveness of early/late pro-B from BM.56 Our results extend these findings by showing that deletion of the CEBPβ gene leads to a decrease in both IL-7 production and IL-7 responsiveness. These findings indicate that a defect in B-lymphocyte development can result from a functional deletion of a cytokine or of its receptor, as well as of a gene whose product acts downstream of the signal transduction pathway. A similar feature has been reported in the case of severe immunodeficiency resulting from inactivation of either the common γ-chain of IL-2, IL-4, and IL-7, or of Jak3 kinase.57-60
Although C/EBPβ-deficient mice present a lymphoid proliferation in spleen and lymph node, humoral and cellular immunity are profoundly distorted as shown by the increased susceptibility to Candida albicans infection and the altered T-helper function.16 Recently it has been reported that C/EBPβ-deficient mice were highly susceptible to infection by Listeria monocytogenes and that the tumor cytotoxicity of macrophages was severely impaired in C/EBPβ-deficient mice.61 These findings, together with the data shown in this study, point to a central role of C/EBPβ in the functional regulation of lymphoid compartments and in macrophage activation, and indicate C/EBPβ −/− mice as a suitable model in immunity and inflammation.
ACKNOWLEDGMENT
We thank M. Brian and J. Foxwell for providing the CT6 cell line and N. Rice for the gift of 70Z/3 cells. We are grateful to R. Taub and L. Greenbaum for the generous gift of C/EBPβ−/− and wild-type mice, and K. Calame for providing the anti C/EBPγ antiserum. We also thank G. Ciliberto for critical reading of the manuscript.
Supported by grants from the Associazione Italiana per la Ricerca sul Cancro (A.I.R.C.), from the Consiglio Nazionale delle Ricerche (C.N.R), from A.I.D.S. project of the Istituto Superiore di Sanità, and from Telethon. X.C. was supported by a fellowship from Telethon.
Address reprint requests to Giuseppe Scala, MD, Dipartimento di Biochimica e Biotecnologie Mediche, Via S. Pansini 5, 80131, Napoli, Italy.

![Fig. 5. Proliferative response of BM B-lymphocyte subpopulations of C/EBPβ+/+ and C/EBPβ−/− mice to IL-7 stimulation. (A) FACS analysis of sorted B-cell subpopulations. BM cells from C/EBPβ+/+ and C/EBPβ−/− mice were stained with CD45/B220-FITC and with antimouse IgM-biotin followed by streptavidin-PE labeling. Cells were sorted in CD45/B220+/IgM− and CD45/B220+/IgM+ cell subsets by FACS sorting, as detailed in Materials and Methods. A purity of greater than 99.5% was consistently achieved. (B) Response of BM B lymphocytes from C/EBPβ+/+ or C/EBPβ −/− mice to IL-7 stimulation. CD45/B220+/IgM− and CD45/B220+/IgM+ cell subsets were isolated from BM cells of C/EBPβ+/+ and C/EBPβ −/− mice, cultured into 96-well plates at 2 × 104/0.1 mL, and stimulated with the indicated amounts of IL-7. On day 7, cultures were obtained to determine [3H]-thymidine incorporation. The data are expressed as the mean ± the standard deviations of three independent experiments. Similar results were obtained in the case of several independent experiments with B-cell populations obtained from long-term B-cell cultures (not shown).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/90/1/10.1182_blood.v90.1.156/5/m_bl_0004f5a.jpeg?Expires=1763530063&Signature=RNVhUOiLh55QFtCzoJm3paA-e6YD63R3CiE~JcPyKEPP4TLJHt4oKgTL-g3snzMJBypSdjWkOB72QlZOyj1A0a0Edsv2SpwvFQf0cnqfBsOkUaEly2NXJAoCmc4IfHV7IYtZjpAaiv03XROF4Tf-ijvsYXsNd0siz9c8vPuWpV915w~kmgBo6ysGKFql7UPauIFgY1eDQCYnqA0fjonTYC8infXIIu-10W-MiYy4pzAF9eTx2y-7jjyZ11chbsJD25Hnhn85lnhhaGgTzIw~kO2LEq8yHn6NDt4WxeKt~5cdgJ0pPXv5aDniS9wWMrK0-1rdZvNxlUUIxYI1MQHYcg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 5. Proliferative response of BM B-lymphocyte subpopulations of C/EBPβ+/+ and C/EBPβ−/− mice to IL-7 stimulation. (A) FACS analysis of sorted B-cell subpopulations. BM cells from C/EBPβ+/+ and C/EBPβ−/− mice were stained with CD45/B220-FITC and with antimouse IgM-biotin followed by streptavidin-PE labeling. Cells were sorted in CD45/B220+/IgM− and CD45/B220+/IgM+ cell subsets by FACS sorting, as detailed in Materials and Methods. A purity of greater than 99.5% was consistently achieved. (B) Response of BM B lymphocytes from C/EBPβ+/+ or C/EBPβ −/− mice to IL-7 stimulation. CD45/B220+/IgM− and CD45/B220+/IgM+ cell subsets were isolated from BM cells of C/EBPβ+/+ and C/EBPβ −/− mice, cultured into 96-well plates at 2 × 104/0.1 mL, and stimulated with the indicated amounts of IL-7. On day 7, cultures were obtained to determine [3H]-thymidine incorporation. The data are expressed as the mean ± the standard deviations of three independent experiments. Similar results were obtained in the case of several independent experiments with B-cell populations obtained from long-term B-cell cultures (not shown).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/90/1/10.1182_blood.v90.1.156/5/m_bl_0004f5b.jpeg?Expires=1763530063&Signature=wkaZQJllBsEwd44UfxlfGlvIabCHj8XL~aaoc43fy3nqsTIc2QEJoIBDx4BjLvSRdBzVv2HxkpWxbAB3gqgACcVnmO68MNE0Dgao1SUSSMte63jECMZUs33b8caD6XiYwtZCMWDOC9WoOlPMaHjYGuXx6aRmBKdIFAwgt39t-JVL5DHXJ6k5wHsN86~KhIKkzEa2q5oSE~aEcZzNXoW~VAHLjxcuqPgH1meK~RnC-SOKwfcQP2xPIL6H1sCu25OdHPbWGO9OmAG7qYYDlte0VuWKikODVX~h3d9d0l8kBji59yx4PfQMwIer-get7aD34dnz4AfGpKjwfGUs1aNSXg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 7. Induction of C/EBP binding activities by IL-7. BM B220+/IgM− pre-B cells were isolated by FACS sorting as detailed in Materials and Methods. BM pre-B lymphocytes and 70Z/3 pre-B cells were stimulated with IL-7 (10 ng/mL) for 2 hours or left untreated. Nuclear extracts were prepared and incubated with [32P]-labeled C/EBP double-strand oligonucleotide, as described in Materials and Methods. Unlabeled competitor probe was used at 100-fold molar excess. One milliliter of the indicated anti-C/EBP antisera or of preimmune rabbit serum was added to the reaction mixtures. The results of a single experiment are shown as a representative of four other independent experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/90/1/10.1182_blood.v90.1.156/5/m_bl_0004f7.jpeg?Expires=1763530063&Signature=I6VCj3-I7Oaiz9G1tTEHbvnAggnm3gDgXYaLf8~2j7HPrbc5aXVLMtxvNwJo~XCfZtqXzTECMh9oXkOYITRH94fsO0WMbmefbyrLS6OuVnrZlNpN~aBNGooK20ZQhh6ADkeVC2w5z8sffsB-l-WIb229CZbPuetOK05vBKlbNLFW8qBUbG2fayxrn7Oo5J2o6TGExMSa9xtfifW-v6M~RGCEbBlDqriLG0ACtkyoEdsRdZD0OqCo9Qoc2M5mxI3PBdYUf3jONHOWsQMTbVdsk~nS0Iq~E4nH3NvmznF6trakwpBFzYFoaFqTwWZtsNF-L95cN0qGV92TFKOfE5QAOA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal