Abstract
Defects in genes involved in DNA mismatch repair have been detected in both hereditary and sporadic tumors of colon, endometrium, and ovary and suggested to be associated with tumorigenesis. To investigate disruptions of the mismatch repair system in hematological malignancies, we examined alterations of the human mutL homologue 1 (hMLH1) gene, a member of the mismatch repair gene family, in a total of 43 human leukemia and lymphoma cell lines, by polymerase chain reaction–single-strand conformation polymorphism (PCR-SSCP) and sequencing analyses. Mutations of the hMLH1 gene were detected in three cell lines established from lymphoid leukemias. Moreover, Northern and Western blot analyses showed that expression of hMLH1 transcript or protein was abrogated in these three leukemia cell lines. Further studies for microsatellite loci showed that these cell lines without hMLH1 expression showed microsatellite instability. This is the first report that describes mutations and inactivation of the hMLH1 gene in human leukemia cells, suggesting that disruption of DNA mismatch repair system may play an important role in the development of human lymphoid leukemias.
SEVERAL MECHANISMS through which cells are subjected to malignant transformation have been identified. Among these, defects in DNA mismatch repair systems have been recently described in colon tumorigenesis related to hereditary nonpolyposis colorectal cancer (HNPCC).1-5 HNPCC is an autosomal dominant disorder and one of the most common genetic conditions that determines susceptibility to colorectal cancers and tumors in other organs, including endometrium, stomach, ovary, ureter, renal pelvis, pancreas, biliary tree, skin, larynx, and bone marrow.6 Recently, in more than 90% of HNPCC kindreds, a germ-line mutation was shown to occur in one of a group of genes involved in DNA nucleotide mismatch repair, including human mutS homologue 2 (hMSH2),7-9 human mutL homologue 1 (hMLH1),10,11 and human postmeiotic segregation 1 (hPMS1) and 2 (hPMS2).12 Examinations on mismatch repair genes in tumors of the HNPCC patients have shown abnormalities in both parental alleles, implying that inactivation occurs in the wild-type allele in addition to the germline mutation.8 12-14
Tumors in HNPCC patients show a particular form of genetic instability, also termed replication error (RER) or microsatellite instability, which is generally characteristic of a mismatch repair defect.2,4,15,16 This results in accumulation of changes in the length of microsatellite and other short-repeat sequences and possibly of single-base changes due to failure to correct mistakes that may occur during DNA replication,17 implying that defects in the mismatch repair system may cause the accumulation of mutations in key oncogenes or tumor suppressor genes, thus contributing to the development of tumors.2,7 8
In addition to HNPCC,2,15,18 microsatellite instabilities have also been observed in a variety of sporadic cancers, including colon, gastric, pancreatic, endometrial, ovarian, and small cell lung carcinomas,19 and in hematopoietic malignancies such as blastic crisis of chronic myelogenous leukemia (CML),20 myelodysplastic syndrome (MDS),21 chronic lymphocytic leukemia (CLL),22 Burkitt's lymphoma and human immunodeficiency virus (HIV)-associated lymphomas.23,24 On the other hand, it is known that lymphoid leukemias and lymphomas sometimes arise in the HNPCC patients.25-27 Of interest, one branch of HNPCC families has developed an excess of hematopoietic tumors.28 From these observations, it is likely that a common defect in the mismatch repair system could affect hematopoietic cells and contribute to the development of leukemias and/or lymphomas.
In this study, we performed mutation analyses of the hMLH1 gene in human hematopoietic tumor cell lines and analysis for microsatellite instability to clarify a possible involvement of aberrant DNA mismatch repair mechanisms in the generation of human leukemias and/or lymphomas.
MATERIALS AND METHODS
Cell lines and preparation of samples.Forty-eight human leukemia and lymphoma cell lines were included in this study consisting of 11 myelocytic/monocytic (HL60, SKH1, KG1, KU812, ME-F2, JOSK-K, JOSK-S, P39 TSU, THP1, U937, and ML1), 4 erythroid (F36E, K562, HEL, and TF1), 5 megakaryocytic (UT7, CMK, MegJ, MOLM1, and SUM90-7), 17 B-lymphocytic (BALL-1, Daudi, Raji, IM9, M5, HA, P32 ISH, SCMCL-L1, SCMCL-L3, SCMCL-L4, KPMM1, KCL22, Ramos, BALM1, Ri-1, RPMI8226, and DND39), and 11 T-lymphocytic cell lines (Jurkat, A3/KAW, MOLT4, MOLT16, P30OHK, SKW3, CEM, MT1, HUT78, Peer, and TALL1). The cells were grown in suspension culture in RPMI medium 1640 supplemented with 10% fetal calf or bovine serum. TF1, UT7, CMK, and F36E are factor-dependent cell lines and were cultured in the presence of 5 ng/mL recombinant human granulocyte-monocyte colony-stimulating factor (rhGM-CSF ). Genomic DNA and total RNA of cells was extracted as described previously.29
Polymerase chain reaction-single–strand conformation polymorphism (PCR-SSCP) and sequencing of the hMLH1 gene.We analyzed the status of the four exons (exons 2, 9, 15, and 16) of the hMLH1 gene in which more than two mutations had been described in previous reports.30-32 Point mutations, small nucleotide deletions, and insertions in these exons were examined by PCR-SSCP and sequencing analyses according to the previously described methodology.33 Nucleotide sequences of the primers used to amplify exons 2, 9, and 16 and conditions of PCR-SSCP analysis were referred to the literature.32 The sequences of the primers used to amplify exon 15 are as follows: 5′-CAGTGAAGAACTGTTCTACCAG-3′ and 5′-GATATTAGTGGAGAGCTACTAT-3′. PCR products that showed polymorphic bands were subcloned into the pCRTMII Vector (Invitrogene, San Diego, CA) and eight clones were sequenced in both directions to confirm mutations.33
Northern blot analysis of hMLH1.Total RNA prepared from cultured cells was separated by electrophoresis through 1.2% agarose-formaldehyde gels (10 μg/lane), blotted onto a nylon membrane, and hybridized with combined hMLH1 probes obtained by PCR amplification of exons 2 and 19 of the gene. Nucleotide sequences of the primers used to amplify exon 19 were also referred to the literature.32 As an internal gauge of RNA level, the blot was also probed with a full-length glyceraldehyde-3-phosphate dehydrogenase (GAPDH) cDNA.
Immunoblotting.Cells were lysed on ice with the lysis buffer containing 50 mmol/L Tris-HCl, pH 7.4, 150 mmol/L NaCl, 0.05% sodium dodecyl sulfate (SDS), 1% deoxycholate, 1% Triton X-100, 10 U/mL aprotinin and 2 mmol/L phenylmethylsulfonyl fluoride. One hundred micrograms of protein was separated through SDS-polyacrylamide gel electrophoresis (PAGE) and electrotransferred onto polyvinylidene difluoride filters (Millipore, Bedford, MA). Filters were probed with both anti-hMLH1 (PharMingen, San Diego, CA) and anti-Hsp 70 (BioMakor, Rehovot, Israel), the latter being used for confirmation of integrity of the protein samples, as previously described.33 The membrane was then washed three times with the Tris-buffered saline with 0.1% Triton X-100 (TBST) buffer containing 10 mmol/L Tris-HCl, pH 7.4, 150 mmol/L NaCl, 0.1% Triton X-100, and incubated at room temperature with an antimouse (IgG1) antibody conjugated to alkaline phosphatase. The specific bands were visualized by calorigenic substrates, nitroblue tetrazolium, and 5-bromo–4-chloro–3-indoyl phosphatase (Promega, Madison, WI).
Assay for microsatellite instability.The techniques used for determining RER status of cell lines are the same as described previously.1-3,19 Briefly, the four microsatellite regions containing repeat sequences, D2S123, D18S58, BAT25, and BAT26, were PCR-amplified using genomic DNAs of the cell lines with hMLH1 alterations. The primers used for these microsatellite markers and conditions for PCR were referred to the literature.34 After PCR amplification, the products were denatured and analyzed on a 6% polyacrylamide denaturing gel containing 8.3 mol/L urea. We tested both DNAs extracted from original cell lines and those from single cell-derived subclones of each cell line isolated by limiting dilution in 96-well microtiter plates.1 19
RESULTS
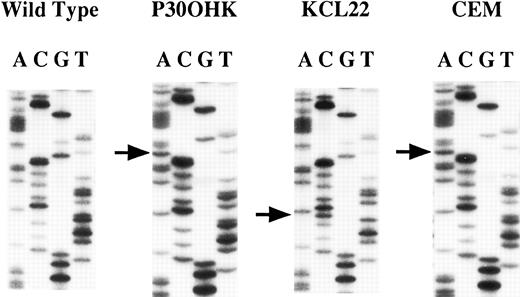
Mutations of the hMLH1 gene.We screened the 43 cell lines for mutations in regions containing exons 2, 9, 15, and 16 of the hMLH1 gene by PCR-SSCP and sequencing analyses and confirmed loss of hMLH1 expression by Northern and/or Western blot analyses for those cell lines that had a mutation of the hMLH1 gene. As for mutations of the exon 9-containing region, abnormally migrating bands were detected on PCR-SSCP analysis in three cell lines; CEM, KCL22, and P30OHK (Fig 1). Sequencing analysis showed nucleotide alterations of the hMLH1 gene in all three cell lines (Fig 2). CEM and KCL22 were thought to be heterozygous for the mutations because both the normal and the mutated sequences of the exon 9–containing region were obtained (data not shown), confirming to the results from the PCR-SSCP analysis, in which normally migrating bands, as well as abnormally migrating bands, were also detected (Fig 1). On the other hand, in P30OHK, the PCR-SSCP analysis showed exclusively abnormally migrating bands and only the abnormal sequence of the exon 9-containing region was obtained from sequencing of the PCR product, suggesting that an allelic loss of the exon 9-containing region exists in this cell line. P30OHK and CEM had the same splicing-site mutation in splicing donor site of intron 9 (Fig 2 and Table 1), while KCL22 had a missense mutation at codon 259 (TTA to TCA) (Fig 2 and Table 1). Results are summarized in Table 1. All of these three cell lines were established from leukemias of lymphoid origin; CEM from T-lineage acute lymphocytic leukemia (T-ALL),35 P30OHK from preT-lineage ALL (PreT-ALL),36 and KCL22 from B-lymphoid crisis of CML.37 38 As summarized in Table 2, mutations were found in 1 of the 12 B-lymphocytic cell lines (8.3%), 2 of the 11 T-lymphocytic cell lines (18.2%), and none of the 20 cell lines of nonlymphoid origins. Mutations were not detected in the regions containing exons 2, 15, and 16 of the hMLH1 gene we examined.
PCR-SSCP analysis of exon 9 of the hMLH1 gene. Lanes 1 through 8 were DNA samples from ML1, TALL1, P30OHK, A3/KAW, CEM, M5, SUM90-7, and KCL22 cell lines, respectively. Lanes 3, 5, and 8 showed abnormal migrating bands.
PCR-SSCP analysis of exon 9 of the hMLH1 gene. Lanes 1 through 8 were DNA samples from ML1, TALL1, P30OHK, A3/KAW, CEM, M5, SUM90-7, and KCL22 cell lines, respectively. Lanes 3, 5, and 8 showed abnormal migrating bands.
Sequencing analysis of the hMLH1 mutations. The left panel represents the normal sequence of the hMLH1 exon 9. The arrows show the positions of point mutations. A nucleotide change G to A in consensus splice donor site is seen in both P30OHK and CEM. A transition of T to C at codon 259 is found in KCL22.
Sequencing analysis of the hMLH1 mutations. The left panel represents the normal sequence of the hMLH1 exon 9. The arrows show the positions of point mutations. A nucleotide change G to A in consensus splice donor site is seen in both P30OHK and CEM. A transition of T to C at codon 259 is found in KCL22.
Expression of the hMLH1 gene.To evaluate expression of hMLH1 transcript and protein, we performed Northern and Western blot analyses, respectively. As shown in Fig 3, a 2.7-kb transcript was detected in all cell lines, except P30OHK and KCL22, in which the hMLH1 message was undetectable despite normal expression of GAPDH. Next, we studied expression of hMLH1 protein in the three cell lines having mutations of the hMLH1 gene. Representative blots are shown in Fig 4. hMLH1 and Hsp70 proteins from normal lymphocytes were observed around 85 and 70 kD, respectively (Fig 4, lane 1). Expression of Hsp70 was used as a control for the amount and integrity of the sample protein. P30OHK, KCL22, and CEM, showed loss of or remarkably low levels of hMLH1 protein expression despite normal expression of Hsp70. The results of expression analyses of hMLH1 are summarized in Table 2.
Northern blot analysis of hMLH1 gene transcript. Filters were hybridized with either hMLH1 probes (upper panel) or GAPDH probe (lower panel) as a control. (A) Lanes 1 through 9 are MegJ, P30OHK, BALL-1, MOLT4, MOLT16, CEM, Raji, THP1, and SKW3, respectively. (B) Lanes 1 through 12 show Ramos, Jurkat, HA, DND39, BALM1, ML1, KCL22, Ri-1, Daudi, HL60, RPMI8226, and TF1, respectively. A 2.7-kb transcript is detectable in all lanes except lane 2 of (A) and lane 7 of (B). No hMLH1 mRNA expression is found in P30OHK and KCL22.
Northern blot analysis of hMLH1 gene transcript. Filters were hybridized with either hMLH1 probes (upper panel) or GAPDH probe (lower panel) as a control. (A) Lanes 1 through 9 are MegJ, P30OHK, BALL-1, MOLT4, MOLT16, CEM, Raji, THP1, and SKW3, respectively. (B) Lanes 1 through 12 show Ramos, Jurkat, HA, DND39, BALM1, ML1, KCL22, Ri-1, Daudi, HL60, RPMI8226, and TF1, respectively. A 2.7-kb transcript is detectable in all lanes except lane 2 of (A) and lane 7 of (B). No hMLH1 mRNA expression is found in P30OHK and KCL22.
Western blot analysis of the hMLH1 protein. hMLH1 protein is observed around 85 kD. The lower bands detected around 70 kD shows expression of Hsp70, as controls for the amounts and integrity of protein. Lane 1 represents normal lymphocytes derived from a normal volunteer used as a normal control. Lanes 2 through 7 are cell lysates prepared from cell lines MOLT16 (lane 2), P30OHK (lane 3), CEM (lane 4), KCL22 (lane 5), BALL1 (lane 6), and U937 (lane 7). P30OHK, CEM, and KCL22 had loss of hMLH1 protein expression with normal expression levels of Hsp70.
Western blot analysis of the hMLH1 protein. hMLH1 protein is observed around 85 kD. The lower bands detected around 70 kD shows expression of Hsp70, as controls for the amounts and integrity of protein. Lane 1 represents normal lymphocytes derived from a normal volunteer used as a normal control. Lanes 2 through 7 are cell lysates prepared from cell lines MOLT16 (lane 2), P30OHK (lane 3), CEM (lane 4), KCL22 (lane 5), BALL1 (lane 6), and U937 (lane 7). P30OHK, CEM, and KCL22 had loss of hMLH1 protein expression with normal expression levels of Hsp70.
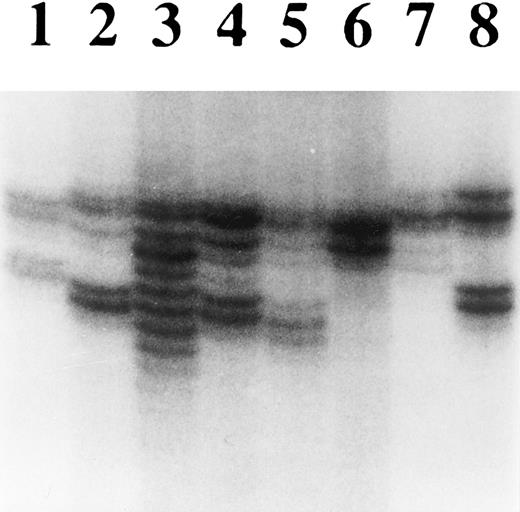
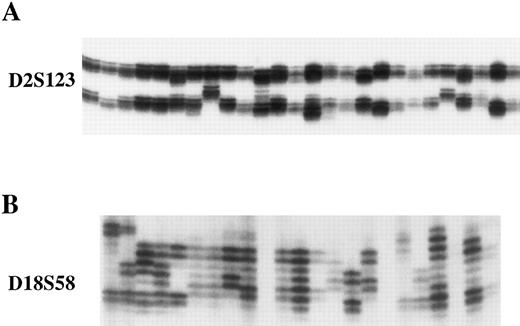
Microsatellite instability in cell lines with loss of hMLH1 expression.To confirm functional loss of hMLH1, the cell lines without hMLH1 protein expression were analyzed for microsatellite instability at four different polymorphic loci. First, we screened P30OHK, KCL22, and CEM for heterogeneity of microsatellite structure. As shown in Fig 5, multiple alleles of microsatellite locus were evident in these three cell lines at D18S58. PCR-amplified allelic markers of P30OHK, KCL22, and CEM all have radiated into tightly bunched ladders of bands that are similar to those originally described in tumors arising in affected members of HNPCC families,2 at a minimum of three of four microsatellite loci. Secondly, to examine microsatellite instability in greater detail, we isolated various single cell clones of KCL22 and CEM. Each single cell clone of KCL22 and CEM was found to exhibit expansions and/or contractions of microsatellite length at all four microsatellite loci. Representative results of microsatellite analysis for single cell clones of KCL22 at D2S123 and D18S58 are shown in Fig 6A and B, respectively (data not shown for CEM). A single cell of P30OHK cell line did not grow well in culture.
Analysis of microsatellite instability of hematopoietic tumor cell lines. DNA purified from each cell line was analyzed for heterogeneity at the microsatellite locus D18S58. Lanes 1 through 8 are normal lymphocytes, U937, KCL22, CEM, P30OHK, HEL, HL60, and SKH1, respectively. Multiple alleles were particularly evident in KCL22, but could also have appeared in CEM and P30OHK.
Analysis of microsatellite instability of hematopoietic tumor cell lines. DNA purified from each cell line was analyzed for heterogeneity at the microsatellite locus D18S58. Lanes 1 through 8 are normal lymphocytes, U937, KCL22, CEM, P30OHK, HEL, HL60, and SKH1, respectively. Multiple alleles were particularly evident in KCL22, but could also have appeared in CEM and P30OHK.
Representative results of analysis of microsatellite instability in single cell clones of KCL22. Single cell clones of KCL22 exhibit microsatellite instability at D2S123 (A) and D18S58 (B). Each lane represents an independent subclone. DNA samples were prepared from single cell clones isolated by limiting dilution, and polymorphic microsatellite sequences were amplified by PCR and analyzed in denaturing sequencing gels. Subclones of KCL22 display different patterns of laddering at both polymorphic microsatellites.
Representative results of analysis of microsatellite instability in single cell clones of KCL22. Single cell clones of KCL22 exhibit microsatellite instability at D2S123 (A) and D18S58 (B). Each lane represents an independent subclone. DNA samples were prepared from single cell clones isolated by limiting dilution, and polymorphic microsatellite sequences were amplified by PCR and analyzed in denaturing sequencing gels. Subclones of KCL22 display different patterns of laddering at both polymorphic microsatellites.
DISCUSSION
We have shown mutations and loss of expression of the hMLH1 gene in three of 43 human hematopoietic tumors cell lines. All three cell lines had mutations in exon 9 or in the boundary of exon 9 and intron 9 and showed loss of hMLH1 protein. Two of the three cell lines, CEM and P30OHK, had the same mutation at 5′-splicing donor site in intron 9. P30OHK showed exclusively abnormal sequence in this position, which most probably resulted from an allelic loss of exon 9 and a point mutation on the residual allele. Because no hMLH1 transcript could be observed in this cell line, it is suggested that the mutation at 5′-splicing donor site of hMLH1 intron 9 might cause abnormal splicing of the transcript and lead to loss or remarkably low level of mature hMLH1 messages. On the other hand, CEM was found to be heterozygous for this splicing donor site mutation; it had both the normal and the mutated sequences of the exon 9 containing region. Because the mutation was the same as that found in P30OHK, no mature hMLH1 transcript could be transcribed from this mutated allele. In addition, the remaining allele is also presumed to become inactivated, because hMLH1 protein was not expressed in CEM. Considering that a full-length hMLH1 message was apparently expressed in CEM, the loss of hMLH1 protein might be ascribed to another mutation in the other exon that we examined.
The other cell line, KCL22, lost hMLH1 transcript. It had an allelic mutation in exon 9, a TTA to TTC transversion. This is a novel mutation that might potentially accompany an amino acid substitution of Leu to Ser. None of the DNA samples from 150 unrelated individuals we examined showed the same nucleic acid substitution. Nevertheless, it might represent either a polymorphism or a mutation of no consequence, because this single mutation itself is not likely to result in total loss of hMLH1 transcript, as shown in KCL22. The loss of hMLH1 transcript in this cell line should thus be ascribed to biallelic mutations somewhere else in the hMLH1 gene.
Mutations and inactivation of mismatch repair genes have been considered as the cause of microsatellite instability phenotype and proposed to foster a mutator phenotype important in the development of cancers.7,8,11,12 Mismatch repair gene defects including hMLH1 and hMSH2 were reported in a part of both sporadic and hereditary colorectal cancers and tumor cell lines with microsatellite instability.13,34,39 Moreover, recent studies have shown that mice engineered to be deficient in MLH1, MSH2, or PMS2 showed microsatellite instability.40-44 These studies clearly show that the RER phenotype is the result of the lack of mismatch repair proficiency. In our analysis, the three cell lines with hMLH1 inactivation showed microsatellite instability, which seems consistent with the above observations. Although there is the possibility of further defects in other mismatch repair genes that we have not studied, inactivation of the hMLH1 gene would result in microsatellite instability in these cell lines.
Several studies showed that microsatellite instability was associated with the development, rather than the initiation, of the carcinoma because microsatellite instability was frequently detected in advanced stage, rather than in the early stage, of colon and gastric cancers.1,4,45-48 In hematological tumors, microsatellite instability in CML patients was observed in cases of the blastic crisis and accelerated phase, but no cases in the chronic phase.20 Viewed in this light, our result that the KCL22 cells, derived from patients in the blastic crisis of CML showed RER phenotype may be of interest.
In our study, mutations of the hMLH1 gene were detected in lymphoid leukemias only. Mutations have not been found in nonlymphoid tumor cell lines, although we could not rule out a possibility that there may be other mutations in some exons of the hMLH1 gene, which we have not studied. With respect to this point, development of hematological malignancies in human HNPCC kindreds was also reported exclusively in lymphoid malignancies including acute and chronic lymphocytic leukemias and malignant lymphomas.25-28 Moreover, the predisposition to lymphoid tumors was reported in MSH2- and PMS2-knockout mice.42-44 Although MLH1-deficient mice were not yet old enough to determine cancer susceptibility and were not fully analyzed, one group recently reported that an MLH1-deficient mouse had developed a lymphoma.40 These observations suggest that defects in these mismatch repair genes, including hMLH1, may be associated with lymphoid malignancies.
In summary, we have detected inactivations of the hMLH1 gene in three of 43 hematopoietic tumor cell lines. This is the first report describing mutations of the hMLH1 gene in human leukemias. Our findings imply that inactivations of the hMLH1 gene may play an important role in the development of human lymphoid tumors. Further studies of hMLH1 and other mismatch repair genes in primary hematological malignancies would be of interest.
ACKNOWLEDGMENT
We gratefully acknowledge Drs Hideaki Mizoguchi, Department of Medicine, Tokyo Women's Medical College, Tokyo, Japan for providing MegJ cell line, Yasuhide Hayashi, Department of Pediatrics, University of Tokyo, Tokyo, Japan for providing SCMCL-L1, SCMCL-L3, and SCMC-L4, Chihiro Shimazaki, Department of Medicine, Kyoto Prefectural University of Medicine, Kyoto, Japan for providing KPMM1, Shigeru Fujita, Department of Medicine, Ehime University, Ehime, Japan for providing ME-F2, and Jun Minowada, Fujisaki Cell Center, Hayashibara Biochemical Laboratories Inc, Okayama, Japan for providing MOLM1, Ramos, BALM1, Ri-1, RPMI8226, DND39, A3/KAW, MOLT16, and SKW3.
Address reprint requests to Hisamaru Hirai, MD, The Third Department of Internal Medicine, Faculty of Medicine, University of Tokyo, 7-3-1 Hongo, Bunkyo-ku, Tokyo 113, Japan.