Abstract
Apoptosis is well known to be mediated by oxidative stress. To evaluate the functional role of reactive oxygen intermediates (ROI) produced by neutrophils, we compared the rates of apoptosis in neutrophils isolated from normal donors and from patients with chronic granulomatous disease (CGD), a hereditary defect in ROI production. Spontaneous cell death in CGD neutrophils in vitro was significantly inhibited relative to normal neutrophils. The acceleration of apoptosis induced by anti-Fas monoclonal antibody (MoAb) in CGD neutrophils was much slower than that seen in normal neutrophils. These findings suggest that the apoptosis of neutrophils may be mediated by endogenous oxidative products. This suggestion was confirmed by observation that apoptosis of normal neutrophils was markedly inhibited by reduction of intracellular levels of hydrogen peroxide (H2O2 ). The inhibition of apoptosis in normal neutrophils by adding catalase occurred regardless of the presence of anti-Fas MoAb. H2O2 increased both spontaneous apoptosis and Fas-mediated apoptosis of the CGD neutrophils in proportion to that seen in normal neutrophils. Although several factors that mediate the apoptosis of neutrophils remain to be determined, these results suggest that ROI are major mediators of the apoptosis in neutrophils and may be involved in Fas-mediated signal transduction pathway.
HUMAN NEUTROPHIL granulocytes play the important role in the host defenses against the invasion of microorganisms. The respiratory burst activity of phagocytes is the essential metabolic events to the killing of ingested microbes by the reactive oxygen intermediates (ROI) generated by the multicomponent enzyme system of membrane-associated NADPH oxidase.1 The functional significance of this process were shown in several studies of patients with chronic granulomatous disease (CGD). The latter is a group of rare hereditary disorders characterized by a diminished or absent production of ROI due to a defect in any one of the components of the NADPH oxidase.2,3 Patients with CGD suffer from severe, recurrent bacterial and fungal infections due to the impaired ability of their granulocytes to kill such microorganisms. Patients with CGD also develop chronic inflammatory granulomas at various sites and have widespread noninfectious complications subsequent to tissue injury.2 3
The prolonged survival of inflammatory cells can cause chronic tissue damages. Therefore, programmed cell death, or apoptosis, and the subsequent engulfment of the apoptotic cells by phagocytes are important to the rapid resolution of inflammations. This is necessary to avoid unwanted tissue damage caused by activated effector cells, specifically the neutrophils and monocytes.4-7 The morphological features seen in apoptosis, which include membranous blebbing, chromatin condensation, and nuclear fragmentation, are highly conserved in diverse cell types, yet a variety of different intrinsic and extrinsic factors regulate this process.7-10 For example, oxidative metabolites are one such mediator of the apoptosis in various types of cells.9,10 Indeed, gamma irradiation, ultraviolet radiation, and tumor necrosis factor (TNF ) induce apoptosis by generating ROI.7,11-16 Neutrophils actively generate ROI and undergo apoptosis rapidly both in vitro4,5,17 and in vivo.18 19 However, the physiological roles of ROI in the apoptosis of neutrophils is unknown. In this study, we attempted to define the involvement of ROI in the apoptosis of neutrophils by comparing neutrophils isolated from normal individuals with those from patients with CGD that completely lack the ability to produce ROI.
MATERIALS AND METHODS
Reagents.Mouse anti-Fas (CD95) monoclonal antibody (MoAb) (CH11, IgM) was purchased from MBL (Nagoya, Japan). Catalase, superoxide dismutase (SOD) and the solution of H2O2 (31% wt/vol) were obtained from Wako Chemicals (Osaka, Japan). Dihydrorhodamine 123 (DHR123: Molecular Probes, Eugene, OR) was dissolved in N,N-dimethyformanide (Sigma Chemical Co, St Louis, MO) at a concentration of 1 mg/mL and stored at 4°C in the dark.
Isolation and culture of human neutrophils.Neutrophils were isolated from heparinized venous blood obtained from healthy donors and 5 patients with X-linked gp91-phox-defective CGD by using a combination of dextran sedimentation and centrifugation through a Ficoll-Hypaque gradient as previously described.17 All 5 patients with CGD were diagnosed by using the nitroblue tetrazolium test and Western blot analysis with a rabbit polyclonal anti-gp91-phox antibody,20 kindly donated by Dr S. Imajoh-Imai (Institute of Medical Science, Tokyo University, Tokyo, Japan). Diagnostic flow cytometry using DHR123 were also performed as described elsewhere.21 The isolated neutrophils were suspended in RPMI 1640 medium supplemented with 10% heat-inactivated human AB serum, 2 × 10−5 mol/L 2-mercaptoethanol, 0.3 mg/mL glutamine, 200 U/mL penicillin G, and 10 μg/mL gentamicin. The cells (0.1 mL at 1 × 106 cells/mL) were cultured in 96-well flat-bottomed plates (Corning Glass Works, Corning, NY) at 37°C in a humidified incubator containing 5% CO2 in air for various times, see Results. In certain experiments, neutrophils were incubated with the indicated concentrations of anti-Fas antibody, catalase, SOD, and/or H2O2 from the beginning of culture.
Identification of apoptotic cells.Apoptosis was determined by flow cytometry as previously described.21 Briefly, the cultured cells were washed twice with PBS and treated overnight with hypotonic fluorochrome solution containing 50 μg/mL of propidium iodide, 0.1% Triton X-100 (Sigma), and 0.1% sodium citrate at 4°C in the dark. The resulting fluorescence of individual nuclei was analyzed by using a Cytoron Absolute flowcytometer (Ortho Diagnostic System, Tokyo, Japan).
Assessment of H2O2 production using flow cytometry.H2O2 production by neutrophils was estimated by flow cytometry using DHR123 fluorescent dye.21 Neutrophils (1 × 106 /mL), in medium, were pretreated with 1 mmol/L of DHR123 solution for 5 minutes at 37°C and incubated for 3 hours at 37°C. The cells were then washed with PBS and the resulting fluorescence was analyzed by flow cytometry. The fluorescence intensity of nonspecific staining with DHR123 was obtained with cells incubated with only DHR123 for 5 minutes at 37°C.
DNA electrophoresis.DNA fragmentation in cultured cells was assessed by electrophoresis through agarose gels as described.17 Briefly, 3 × 106 cultured neutrophils were washed twice with PBS and the cell pellet was lysed for 10 minutes on ice in a buffer containing 10 mmol/L Tris-HCl (pH 7.5), 10 mmol/L EDTA, and 0.2% Triton X-100. After removing the nuclei by centrifugation at 13,000 rpm for 10 minutes, DNA was extracted with phenol:chloroform:isoamyl alcohol (25:24:1[V/V]). The extracted DNA was precipitated with 70 % ethanol overnight at −20°C. The samples were air-dried and dissolved in a buffer containing 10 mmol/L Tris-HCl (pH 7.5) and 1 mmol/L EDTA. The samples were then digested with 1 mg/mL RNAse A for 3 hours at 37°C and were electrophoresed through 1.7 % agarose gel and stained with 50 μg/mL ethidium bromide and visualized under ultraviolet light.
Measurements of cytokines and endotoxin.Granulocyte colony-stimulating factor (G-CSF ), granulocyte-macrophage colony-stimulating factor (GM-CSF ), and interleukin-6 (IL-6) concentrations in sera from CGD patients were determined by commercial enzyme-linked immunosorbent assay kit from Medgenix Diagnostics (Fleurus, Belgium) and Fuji-rebio (Tokyo, Japan) respectively. The endotoxin levels in sera were measured by Limulus test at clinical laboratory of Kanazawa University Hospital.
Statistical analysis.Data are expressed as mean ± SD. Differences between data sets were evaluated by performing a unpaired Student's t-test. A level of P < .05 was accepted as statistically significant.
RESULTS
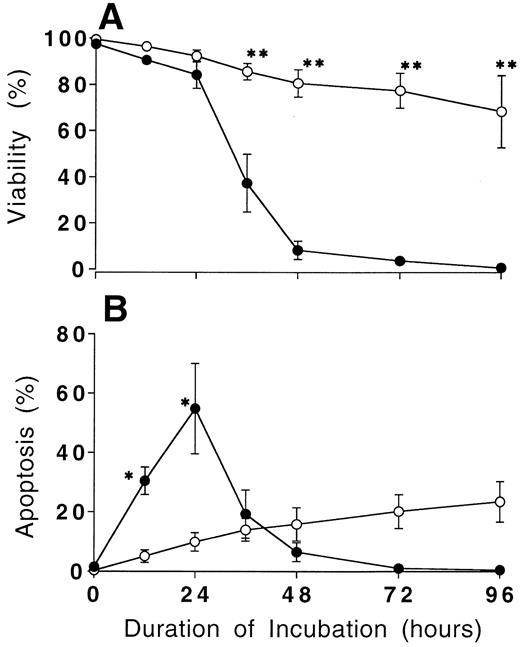
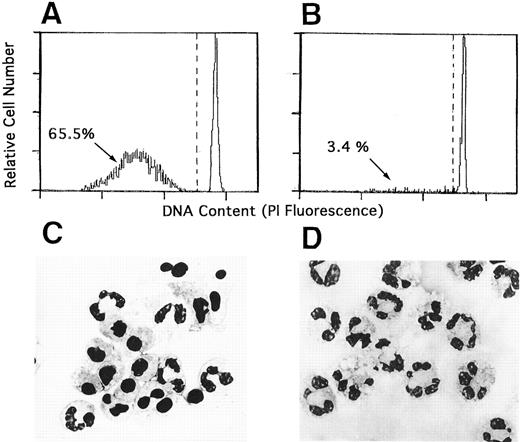
Prolonged survival and defective apoptosis in CGD neutrophils in culture.Trypan blue exclusion revealed that the majority of normal neutrophils cultured in vitro maintained the cell membrane integrity until 24 hours of culture, but after 24 hours of culture normal neutrophils lost cell viability rapidly (Fig 1). Two thirds of the cultured neutrophils obtained from normal donor showed typical apoptotic morphology with the expected decrease in DNA contents. Flow cytometry done at 24 hours of culture revealed these results, which corroborated previously published observation2-5 (Fig 2A and C). In marked contrast, neutrophils from the patients with CGD exhibited little apoptotic change until 24 hours of culture (Fig 2B and D), more than half of them were viable at 96 hours of culture (Fig 1).
Prolonged survival of CGD neutrophils in culture. Isolated neutrophils from normal donors (•) and CGD patients (○) were cultured in vitro. The percentage of cell viability (A) was obtained by using the trypan blue exclusion test, and apoptosis (B) was estimated by flow cytometry as described in Materials and Methods at the indicated times. Data expressed the mean ± SD value obtained from three experiments. *P < .05, **P < .01 versus normal neutrophils.
Prolonged survival of CGD neutrophils in culture. Isolated neutrophils from normal donors (•) and CGD patients (○) were cultured in vitro. The percentage of cell viability (A) was obtained by using the trypan blue exclusion test, and apoptosis (B) was estimated by flow cytometry as described in Materials and Methods at the indicated times. Data expressed the mean ± SD value obtained from three experiments. *P < .05, **P < .01 versus normal neutrophils.
Senescent CGD neutrophils did not show apoptosis in vitro. Neutrophils from normal donors (A and C) and CGD patients (B and D) were cultured in medium for 24 hours. The cells were treated with hypotonic propidium iodide solution and the DNA content was analyzed by flow cytometry (A and B). Morphology of these same cultures is shown for comparison with May-Grünwald-Giemsa–stained preparations photographed at a magnification 1,000× (C and D).
Senescent CGD neutrophils did not show apoptosis in vitro. Neutrophils from normal donors (A and C) and CGD patients (B and D) were cultured in medium for 24 hours. The cells were treated with hypotonic propidium iodide solution and the DNA content was analyzed by flow cytometry (A and B). Morphology of these same cultures is shown for comparison with May-Grünwald-Giemsa–stained preparations photographed at a magnification 1,000× (C and D).
CGD neutrophils are resistant to anti-Fas-MoAb treatments.We have previously shown that the treatment of agonistic anti-Fas MoAb significantly accelerated the apoptosis of neutrophils.17 Thus, we compared the rates of CD95-mediated apoptosis of neutrophils from CGD patients and those from normal donors. Significant differences in apoptotic rates in neutrophils cultured both in the presence and in the absence of anti-Fas MoAb were evident at 12 hours of culture (Table 1). The increase of apoptosis of neutrophils by anti-Fas MoAb was much weaker in the CGD patients, even after 24 hours of culture. Apoptosis of the normal neutrophils was accelerated in a dose-dependent manner when anti-Fas Ab was added to the culture. In contrast, the addition of anti-Fas MoAb to the cultures of CGD neutrophils resulted in only a weak acceleration of apoptosis. Neutrophils from normal donors and CGD patients expressed Fas antigen to an equal extent as determined by flow cytometry (results not shown). Two patients in this study received the treatments with interferon-γ, which reportedly aids their clinical management by controlling infection.23 The results obtained with the neutrophils from these two patients resembled those in other three patients with CGD.
Effect of Anti-Fas MoAb Treatment on Apoptosis of Neutrophils From Normal Donors and CGD Patients
| Source . | Additive . | Amount of Additive (ng/mL) . | Apoptosis (%) After Culture for . | |
|---|---|---|---|---|
| . | . | . | 12 h . | 24 h . |
| Normal | None | 32.5 ± 6.8 | 62.3 ± 12.4 | |
| Control IgM | 300 | 34.2 ± 7.3 | 64.9 ± 10.7 | |
| Anti-Fas | 10 | 37.5 ± 6.2 | ND | |
| Anti-Fas | 100 | 66.7 ± 4.0 | 89.5 ± 4.2 | |
| Anti-Fas | 300 | 85.7 ± 5.1 | 91.5 ± 3.4 | |
| CGD | None | 6.1 ± 1.5* | 10.5 ± 4.1† | |
| Control IgM | 300 | 7.4 ± 1.8* | 11.7 ± 4.9† | |
| Anti-Fas | 10 | 6.9 ± 2.3† | ND | |
| Anti-Fas | 30 | 13.8 ± 3.6† | ND | |
| Anti-Fas | 100 | 16.5 ± 2.9† | 26.2 ± 6.1† | |
| Anti-Fas | 300 | 21.4 ± 3.8† | 35.7 ± 8.4† | |
| Source . | Additive . | Amount of Additive (ng/mL) . | Apoptosis (%) After Culture for . | |
|---|---|---|---|---|
| . | . | . | 12 h . | 24 h . |
| Normal | None | 32.5 ± 6.8 | 62.3 ± 12.4 | |
| Control IgM | 300 | 34.2 ± 7.3 | 64.9 ± 10.7 | |
| Anti-Fas | 10 | 37.5 ± 6.2 | ND | |
| Anti-Fas | 100 | 66.7 ± 4.0 | 89.5 ± 4.2 | |
| Anti-Fas | 300 | 85.7 ± 5.1 | 91.5 ± 3.4 | |
| CGD | None | 6.1 ± 1.5* | 10.5 ± 4.1† | |
| Control IgM | 300 | 7.4 ± 1.8* | 11.7 ± 4.9† | |
| Anti-Fas | 10 | 6.9 ± 2.3† | ND | |
| Anti-Fas | 30 | 13.8 ± 3.6† | ND | |
| Anti-Fas | 100 | 16.5 ± 2.9† | 26.2 ± 6.1† | |
| Anti-Fas | 300 | 21.4 ± 3.8† | 35.7 ± 8.4† | |
Data are expressed as mean percentages ± SD from 4 different experiments.
Abbreviation: ND, not done.
P < .05.
P < .01 versus normal neutrophils.
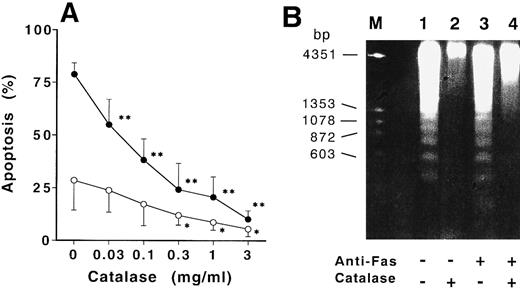
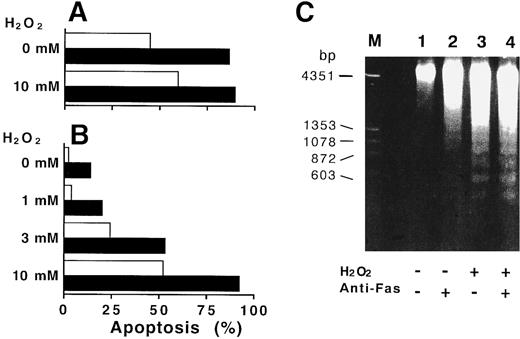
Catalase inhibited the apoptosis in normal neutrophils and H2O2 accelerated the apoptosis in CGD neutrophils.Since ROI production is defective in CGD neutrophils,2,23 the amounts of ROI could influence the differences in apoptotic changes observed in the normal neutrophils and CGD neutrophils. As shown in Fig 3A, exogenous catalase dose-dependently inhibited the spontaneous apoptosis of normal neutrophils after 12 hours of culture. The most striking finding was that the acceleration of the anti-Fas induced–apoptosis was reduced dose dependently by the catalase. The inhibition of apoptosis by catalase was abolished by inactivation of catalytic activity with previous heat-treatment (56.3% ± 4.6% in Fas-treated apoptosis, 24.3% ± 4.9% in spontaneous apoptosis). The inhibition of DNA fragmentation was also confirmed by the electrophoresis of extracted DNA from normal neutrophils cultured for 9 hours (Fig 3B). The addition of N-acetyl-L-cysteine or thioredoxin, ROI scavenger,12 15 SOD did not influence the rates of spontaneous and Fas-induced apoptosis of normal neutrophils (72.5% ± 6.5% in Fas-treated apoptosis, 27.2% ± 8.2% in spontaneous apoptosis). However, the addition of H2O2 to the CGD neutrophil culture led to a significant acceleration in apoptosis at 15 hours of culture. The apoptosis in CGD neutrophils treated with anti-Fas MoAb was increased to a rate comparable to that seen in normal cells (Fig 4).
Catalase inhibited the CD95-induced and spontaneous apoptosis of normal neutrophils. (A) Neutrophils from normal donors were cultured with increasing concentrations of catalase in the presence (•) or absence (○) of 100 ng/mL anti-Fas MoAb for 12 hours and apoptosis of aged neutrophils was then estimated by flow cytometry as described in Materials and Methods. Data are expressed as mean ± SD from 4 different experiments. *P < .05, **P < 0.01 versus neutrophils cultured without addition of catalase. (B) DNA samples extracted from neutrophils cultured for 9 hours with medium alone (lane 1), 1 mg/mL of catalase (lane 2), 100 ng/mL of anti-Fas MoAb (lane 3), or catalase plus anti-Fas MoAb (lane 4) were electrophoresed through 1.7 % of agarose gel as described in Materials and Methods.
Catalase inhibited the CD95-induced and spontaneous apoptosis of normal neutrophils. (A) Neutrophils from normal donors were cultured with increasing concentrations of catalase in the presence (•) or absence (○) of 100 ng/mL anti-Fas MoAb for 12 hours and apoptosis of aged neutrophils was then estimated by flow cytometry as described in Materials and Methods. Data are expressed as mean ± SD from 4 different experiments. *P < .05, **P < 0.01 versus neutrophils cultured without addition of catalase. (B) DNA samples extracted from neutrophils cultured for 9 hours with medium alone (lane 1), 1 mg/mL of catalase (lane 2), 100 ng/mL of anti-Fas MoAb (lane 3), or catalase plus anti-Fas MoAb (lane 4) were electrophoresed through 1.7 % of agarose gel as described in Materials and Methods.
H2O2 -accelerated apoptosis of CGD neutrophils. Neutrophils from normal donors (A) and CGD patients (B) were cultured with various concentrations of H2O2 in the presence (▪) or absence (□) of 100 ng/mL anti-CD95 MoAb. After 15 hours in culture, apoptosis of senescent neutrophils was estimated by flow cytometry described in Materials and Methods. (A) and (B) are representative of three independent experiments. (C) Appearance of fragmented DNA in CGD neutrophils incubated with H2O2 . The nuclear DNA samples extracted from CGD neutrophils cultured for 9 hours with medium alone (lane 1), 100 ng/mL of anti-Fas MoAb (lane 2), 1 mmol/L H2O2 (lane 3), or H2O2 plus anti-Fas MoAb (lane 4) were electrophoresed through 1.7 % of agarose gel as described in Materials and Methods.
H2O2 -accelerated apoptosis of CGD neutrophils. Neutrophils from normal donors (A) and CGD patients (B) were cultured with various concentrations of H2O2 in the presence (▪) or absence (□) of 100 ng/mL anti-CD95 MoAb. After 15 hours in culture, apoptosis of senescent neutrophils was estimated by flow cytometry described in Materials and Methods. (A) and (B) are representative of three independent experiments. (C) Appearance of fragmented DNA in CGD neutrophils incubated with H2O2 . The nuclear DNA samples extracted from CGD neutrophils cultured for 9 hours with medium alone (lane 1), 100 ng/mL of anti-Fas MoAb (lane 2), 1 mmol/L H2O2 (lane 3), or H2O2 plus anti-Fas MoAb (lane 4) were electrophoresed through 1.7 % of agarose gel as described in Materials and Methods.
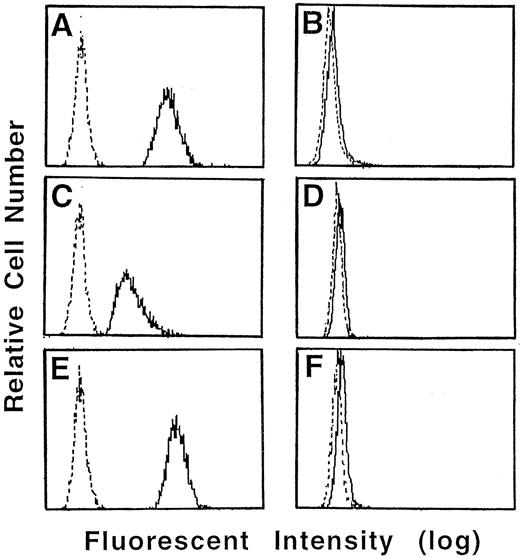
Anti-Fas MoAb did not augment the H2O2 production from neutrophils.To determine whether the Fas-induced acceleration of apoptosis was mediated by a increase in ROI production, we measured the production of H2O2 by highly sensitive method of flow cytometry using DHR123. As shown in Fig 5, even when incubated with medium alone, neutrophils from normal donors produced significant amounts of H2O2 . The production of H2O2 by normal neutrophils increased gradually in culture (results not shown). Conversely, no H2O2 production from CGD neutrophils was detected at any duration of culture. Anti-Fas MoAb treatment did not increase the production of H2O2 by normal neutrophils. The reduction in H2O2 level by catalase was confirmed by demonstration of decrease in the intensity of fluorescence in the neutrophils incubated with catalase. This decrease was related to the concentration of catalase added to the culture (results not shown).
Effect of anti-Fas MoAb and catalase on H2O2 production in cultured neutrophils. Neutrophils from normal donor (A, C, and E) and CGD patients (B, D, and F ) were pretreated with 1 mmol/L of DHR123 for 5 minutes and then cultured with medium alone (A and B) or 1 mg/mL of catalase (C and D) or 100 ng/mL of anti-Fas MoAb (E and F ). After 3 hours in culture, the cells were washed and the fluorescent intensity was examined by Cytoron Absolutes flowcytometer (Ortho Diagnostic System).
Effect of anti-Fas MoAb and catalase on H2O2 production in cultured neutrophils. Neutrophils from normal donor (A, C, and E) and CGD patients (B, D, and F ) were pretreated with 1 mmol/L of DHR123 for 5 minutes and then cultured with medium alone (A and B) or 1 mg/mL of catalase (C and D) or 100 ng/mL of anti-Fas MoAb (E and F ). After 3 hours in culture, the cells were washed and the fluorescent intensity was examined by Cytoron Absolutes flowcytometer (Ortho Diagnostic System).
DISCUSSION
In the present study, we observed that neutrophils from CGD patients, unlike normal neutrophils, were resistant to in vitro apoptosis. Our observations support the results of previous report showing that the apoptosis of netrophils were inhibited under hypoxic culture conditions, which extremely decreased the generation of ROI.24 These results suggest that the apoptosis of neutrophils might be mainly mediated by their oxidative metabolites. This hypothesis was confirmed by the experiments that exogenous H2O2 promoted the apoptosis of CGD neutrophils and that catalase inhibited the apoptosis of normal neutrophils. Since the heat-inactivation of catalase abolished the inhibitory effects of catalase in the apoptosis of normal neutrophils and catalase decreased the intracellular H2O2 levels in cultured neutrophils, the inhibitory effects of catalase in this process is mainly due to the catalytic activity of H2O2 . From the fact that SOD did not influenced the spontaneous and Fas-mediated apoptosis of neutrophils, the superoxide radicals may not play the significant role in apoptosis of neutrophils.
Prolonged cell survival and the inhibition of apoptosis in normal neutrophils was observed when they were treated with bacterial lipopolysaccharide, G-CSF or GM-CSF.25-28 For these reasons, we could not exclude the possibility that the apoptosis of CGD neutrophils was inhibited by a exposure to bacterial or fungal endotoxin in vivo or by a subsequent activation of neutrophils by inflammatory cytokines. Indeed, detectable levels of endotoxin (5 to 8 pg/mL) were found in the sera from our CGD patients without obvious infections. However, the concentrations of G-CSF, GM-CSF, and IL-6 were not elevated in sera from CGD patients (our unpublished data). This suggests that the slower rates of CGD neutrophil apoptosis is independent on pre-existing infection in vivo. Furthermore, we observed that neutrophils from patients with severe bacterial infection revealed only moderate inhibition of spontaneous apoptosis (our unpublished data). The previous reports that the G-CSF treatment did not suppress the membranous oxidative activity of neutrophils and that Fas-induced apoptosis in normal neutrophils was not inhibited by G-CSF17,26 suggest the different anti-apoptotic mechanism of this cytokine other than regulation of ROI activity.
Fas/APO-1 antigen, recently designated CD95, is a member of the TNF- receptor family.29 This antigen is involved in a signal transduction that results in the programmed cell death in a variety of Fas positive cells.30-32 Recent advances in the understanding of Fas and Fas ligand system have now clarified the physiological significance of this system particularly to clonal deletion of activated lymphoid cells in peripheral tissues.32 However, the functional role of ROI in Fas-induced apoptosis was controversial33-36 and the precise signal transduction pathway through which CD95 antigen exerts its control is not fully understood. We found that the neutrophil apoptosis induced by anti-Fas MoAb extremely delayed in the culture conditions which ROI generation was decreased. This suggests that ROI augment the metabolic death effects delivered through CD95 antigen. This notion support the results of previous report that the Fas-induced apoptosis of activated monocytes, another cell population of ROI producing phagocytes, depend on the intracellular level of ROI.33 The existence of ROI independent processes has been described in Fas-mediated cell death.34 The intracellular sites and quantity of ROI produced may influence the experimental results because NADPH oxidase produces large quantity of ROI at either the plasma membrane or the phagosomal membrane of phagocytes, other cells produced them through the cellular respiration at the mitochondrion. For this reason, the antioxidants such as thioredoxin and N-acetyl-L-cysteine, which prevent the apoptosis of lymphoid cells by scavenging ROI but have much weaker antioxidant activity than catalase has,12 15 had no inhibitory effects on the apoptosis of normal neutrophils.
The pathogenesis of CGD is based on the inability of phagocytes to kill microorganism due to the defective production of oxidative metabolites. Since CGD neutrophils hardly undergo apoptosis in vitro, this might contribute to the expansion and deterioration of clinical symptoms and cause the formation of characteristic granulomatous lesions. It is possible that CGD neutrophils might remain alive at sites of inflammation, without undergoing apoptosis, thus leading to an absence of phagocytosis by tissue macrophages. When senescent CGD neutrophils are not being engulfed by macrophages, they release various cytotoxic enzymes and inflammatory cytokines that are harmful to the host. This results in tissue damages, activation, and unnecessary migration of monocytes and neutrophils, to the infection sites. The microbicidal processes of neutrophils are mediated not only by ROI generation but also by several cytosolic proteins including myeloperoxidase, proteases, and other hydrolytic enzymes and these enzymes may influence the rates of apoptosis in neutrophils. Further study is required to understand the role of these enzymes in neutrophil apoptosis, for example with analysis of neutrophils from the patients with myeloperoxidase deficiency.
ACKNOWLEDGMENT
We thank Miho Takamatsu and Chiaki Kato for their technical assistance.
Supported by a Grant-in-Aid for Scientific Research from the Ministry of Education, Science, and Culture of Japan and a grant from the Ministry of Welfare and Health of Japan.
Address reprint requests to Yoshihito Kasahara, MD, PhD, Department of Pediatrics, School of Medicine, Kanazawa University, 13-1 Takaramachi, Kanazawa, Ishikawa 920, Japan.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal