Abstract
Chronic granulomatous disease (CGD) can result from any of four single gene defects involving the components of the superoxide (O−2 ) generating phagocyte nicotinamide adenine dinucleotide phosphate (NADPH) oxidase. We show that transduction of peripheral blood CD34+ hematopoietic progenitors from a p67phox deficient CGD patient with replication defective amphotropic retrovirus encoding p67phox (MFGS-p67phox) significantly corrected the CGD functional defect in phagocyte oxidase activity in vitro. Using a chemiluminescence assay of oxidase activity, we showed that transduced patient CD34+ progenitors differentiating to myeloid cells in culture produced 25% of the total superoxide produced by normal CD34+ progenitors differentiating in culture. A flow cytometric assay of oxidase activity used to assess the oxidase function of individual cells in the cultures indicated that up to 32% of maturing granulocytes derived from transduced CD34+ progenitors from the p67phox CGD patient were oxidase positive with the average level of correction per granulocyte of 85% of that seen with granulocytes in similar cultures of CD34+ progenitors from normal volunteers. Nitroblue tetrazolium dye reduction assays of colonies of transduced progenitors in soft agar indicated that in some studies restoration of oxidase activity occurred in myeloid cells within 44% of granulocyte-erythrocyte-monocyte colonies, and within 28% of the combined group of granulocyte colonies/monocyte colonies/granulocyte-monocyte colonies. These high correction rates were achieved without any selective regimen to enrich for transduced cells. This study provides a basis for development of gene therapy for the p67phox deficient form of CGD.
CHRONIC GRANULOMATOUS diseases (CGD) are a group of four inherited disorders with a common phenotype characterized by a failure of blood phagocytic cells (neutrophils, monocytes, eosinophils) to produce superoxide. This results in a syndrome of recurrent severe pyogenic infections and granuloma formation.1,2 The specific phagocyte nicotinamide adenine dinucleotide phosphate (NADPH) oxidase responsible for this superoxide generation is composed of cytoplasmic and transmembrane protein components that assemble at the cell membrane to form the active phagocyte oxidase enzyme complex after stimulation of the phagocyte.3,4 On cell activation, three cytosolic oxidase components p47phox, p67phox, and rac (a low molecular weight GTPase) translocate to the membrane and bind flavocytochrome b558 , which is composed of oxidase polypeptides gp91phox and p22phox to form an enzymatically active complex.3-8 Four CGD genotypes have been described involving mutations affecting one or another of the phagocyte oxidase protein components of the oxidase enzyme.3,4,9,10 Recently, a sixth protein p40phox has been described, which may also have a role in oxidase assembly.11
CGD is an ideal disease to consider as a target for gene therapy because the cellular functional defect is defined and the CGD genes have been cloned.12-17 Furthermore, for each of the four genetic types of CGD, tissue culture lines derived from patients or engineered to knock out one of the oxidase genes have been used to show that retrovirus or plasmid vectors encoding the appropriate oxidase gene can restore oxidase function.18-27
For the purpose of somatic gene therapy hematopoietic stem/progenitor cells are the ultimate target. Of particular interest are CD34+ peripheral blood hematopoietic progenitor cells (CD34+ PBHP) because this cell population can be mobilized from marrow to the peripheral blood (PB) by administration of granulocyte colony-stimulating factor (G-CSF ), obtained by apheresis, and enriched in clinically relevant amounts by immunoaffinity techniques.28-31 PBHPs share with bone marrow (BM) precursors the ability to achieve hematopoietic reconstitution via infusion of these cells in patients who have had ablative chemotherapy.29 Of relevance to the current study, PBHP appear to be a receptive target for retrovirus mediated gene transfer,22,27,30 suggesting a potential role of PBHP for somatic gene therapy as a substitute for traditional BM transplantation whose inherent risks limit its applicability for CGD patients.32 33 In addition, PBHPs were used in this study as opposed to BM because of their relative ease of harvest and minimal trauma caused to patients.
In previous studies we have used the high-efficiency retrovirus vector, MFGS, encoding either p47phox, p22phox, or gp91phox to achieve significant correction of the functional oxidase defect in phagocytes developed in culture from transduced CD34+ PBHP of patients with the p47phox deficient, p22phox deficient, or gp91phox deficient forms of CGD.22 27 In the present study, we have used an MFGS based retrovirus vector encoding p67phox to correct the oxidase activity of granulocytes differentiated from CD34+ PBHP derived from a p67phox deficient CGD patient.
MATERIALS AND METHODS
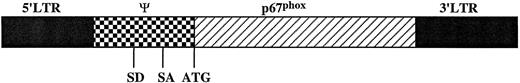
Construction of retrovirus vectors and producer clones.The open reading frame of p67phox cDNA with no flanking sequence was inserted into the Nco I cloning site of the MFGS retrovirus vector (a gift of Somatix Therapy Inc, Alameda, CA).34 MFGS is derived from the previously described MFG vector,34 but contains additional stop codons to interfere with expression of gag sequence. The MFG series of vectors contains no selective markers and was designed to enhance production of recombinant proteins in target tissues by incorporating splicing elements in the ψ-packaging recognition region and a 5′ Nco I cloning site that corresponds to the ATG start of the envelope gene.34-36 MFGS was used to construct MFGS-p67phox (Fig 1), which encodes only the open reading frame of p67phox. MFGS-p67phox was then transfected into ψ-crip amphotropic packaging cells36 to create lines producing infective, but replication defective, MFGS-p67phox amphotropic retrovirus. Generation of the ψ-crip packaging cell clones that produce MFGS-p67phox amphotropic retrovirus was performed as previously described22,27 by transfecting 5 × 105 ψ-crip cells with a mixture of 18 μg of MFGS-p67phox plasmid DNA and 2 μg of PSV2-NEO plasmid DNA using the calcium phosphate precipitation method, then selecting for neomycin resistance with the neomycin analogue G418 (GIBCO-BRL, Grand Island, NY) 2 mg/mL as previously described.22,27,37 Clones were screened for the production of retrovirus capable of transducing expression of p67phox protein in murine National Institutes of Health (NIH) 3T3 fibroblasts using sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) immunoblot analysis.22 Producer clones were maintained in Dulbecco's modified Eagle's medium (DMEM; BioFluids, Rockville, MD) with 10% bovine calf serum (Hyclone, Logan, UT) and 2 mmol/L glutamine (Quality Biological Inc, Gaithersburg, MD) without G418.
Structure of the MFGS-p67phox retroviral vector. The MFGS vector consists of the following parts from left to right in the figure: (A) a Mo-MuLV DNA fragment containing the 5′ LTR sequence; (B) the retroviral ψ-packaging sequence that contains splice donor (SD) and splice acceptor (SA) sites and residual gag sequence with extra stop codons; (C) the open reading frame of p67phox, which was cloned into a unique Nco I site directly 3′ of the retroviral envelope ATG start codon; and (D) a Mo-MuLV 3′ long-terminal repeat (LTR).
Structure of the MFGS-p67phox retroviral vector. The MFGS vector consists of the following parts from left to right in the figure: (A) a Mo-MuLV DNA fragment containing the 5′ LTR sequence; (B) the retroviral ψ-packaging sequence that contains splice donor (SD) and splice acceptor (SA) sites and residual gag sequence with extra stop codons; (C) the open reading frame of p67phox, which was cloned into a unique Nco I site directly 3′ of the retroviral envelope ATG start codon; and (D) a Mo-MuLV 3′ long-terminal repeat (LTR).
Production and use of retrovirus culture supernatants for transduction.Virus supernatants used in all studies were standardized by plating 4 million producer cells in a 75-cm2 flask and culturing to confluency. When the cells were confluent, the media was exchanged for 6 mL of fresh media for 6 hours and collected. The viral supernatant was centrifuged at 400g for 5 minutes to remove cells and debris, and used either fresh or after storage frozen at −70°C. For sham transductions media alone without virus was used.
Purification and culture of PBHP.After obtaining informed consent (NIH protocol 94-I-73) and following 5 daily subcutaneous injections of 10 μg/kg/d G-CSF (Amgen Inc, Thousand Oaks, CA), low-density cells from the PB of normal donors or a patient with p67phox deficient CGD were collected by apheresis as described previously.22,27,38-40 From the apheresis preparation, PBHPs were selected by isolating cells expressing the early hematopoietic cell surface marker, CD34, using the Baxter Isolex system41-44 (Baxter Healthcare, Fenwal Division, Chicago, IL), which uses immuno-magnetic bead technology. For the cells used in this study this selection procedure following manufacturer's specifications, resulted in a product that contained 40% to 90% CD34+ cells. Purified CD34+ cells were frozen (liquid nitrogen vapor phase) in X-Vivo 10 medium (Bio Whittaker, Walkersville, MD) containing 1% human serum albumin (HSA; Baxter Healthcare, Hyland Division, Glendale, CA) and 10% dimethyl sulfoxide (Sigma Chemical Co, St Louis, MO) until use. Before retrovirus transduction, the enriched CD34+ PBHPs were grown in suspension cultures (37°C and 7% CO2 ) for 24 hours, initiated at 0.8 × 106 cells/mL in PBHP growth medium, defined as X-Vivo 10 media supplemented with 1% HSA and three-recombinant human growth factors; 100 ng/mL PIXY321 (interleukin-3/granulocyte-macrophage colony-stimulating factor [IL-3/GM-CSF] fusion protein; a gift from Immunex Corp, Seattle, WA), 100 ng/mL stem cell factor (R&D Systems, Minneapolis, MN), and 10 ng/mL G-CSF (R&D Systems).44-47
Transduction of target cells with MFGS-p67phox retrovirus supernatant.NIH 3T3 cells plated at 500,000 cells were exposed to 2 mL viral supernatant diluted 1:1 with fresh media or only fresh media (sham transduction) in the presence of 6 μg/mL protamine (Elkins-Sinn Inc, Cherry Hill, NJ) in six-well culture plates. Plates were then centrifuged for 60 minutes at 1,200g at 32°C, a maneuver we and others have found to increase transduction rates.48 Following centrifugation, plates were incubated for 5 hours at 37°C and 7% CO2 . Transduction media was then exchanged for fresh DMEM, 10% fetal calf serum (Hyclone) and the NIH3T3 cells were cultured for 3 days. After 3 days, cells were obtained and crude protein fractions from cell lysates were tested for the presence of recombinant p67phox protein by SDS-PAGE immunoblot.22
Transduction of CD34+ PBHP was performed after the initial 24 hours of culture by seeding 0.8 × 106 cells per well into six-well culture plates containing 5 mL of viral supernatant diluted 1:1 with PBHP growth media and 6 μg/mL protamine. Plates were then centrifuged for 60 minutes at 1,200g at 32°C. Following centrifugation plates were incubated for 5 hours at 37°C and 7% CO2 . Transduction media was then exchanged for fresh PBHP growth media. Cells were then incubated overnight and the transduction procedure repeated on a second and third day. Following the third transduction, cells were returned to the standard PBHP growth media and maintained in liquid or plated on semisolid agarose.22,27 Agarose colony cultures also contained erythropoietin (R&D Systems) 2 U/mL, to facilitate development of erythroid colonies and thus allow recognition of granulocyte-erythrocyte-monocyte (GEM) colonies derived from multipotential early progenitors.28 30
Detection of recombinant p67phox protein by Western immunoblot.NIH 3T3 cultures, were collected on day 3 following transduction, and differentiating CD34+ cells were collected on day 17 of culture, and analyzed by SDS-PAGE immunoblot as previously described.17,22,49 Blots were probed with a goat anti-p67phox sera49 at a 1:1,000-fold dilution followed by a secondary antibody peroxidase-conjugated rabbit affinity purified antibody to goat IgG (Organon Teknika Corp, West Chester, PA), and developed using the ECL Western blotting detection kit (Amersham Life Sciences, Arlington Heights, IL) according to manufacturer's recommendations.
Assays of superoxide production by mature myeloid cells derived from PBHP in culture.Nitroblue tetrazolium dye (NBT; Sigma Chemical Co) reduction to formazan precipitate was used as a measure of superoxide production by PBHP myeloid colonies in agarose at 14 days of growth by layering 1 mL of PBS (Dulbecco and Vogt, Quality Biological) 0.2% NBT and phorbol myristate acetate (PMA; Sigma Chemical Co) 1 μg/mL over the culture as previously described.22 After 40 minutes cells were fixed by adding 1.5% paraformaldehyde in buffered saline. NBT positive and negative colonies were counted under an inverted microscope at a magnification of 100×. Only colonies with cells demonstrating intense staining were scored as positive. In control studies this led to intense blue-black staining of the myeloid elements of most colonies derived from normal CD34+ cells, but only faint staining of similar colonies derived from the p67phox deficient CGD patient CD34+ cells.
The products of oxidase activation also were measured in individual cells by flow cytometry using the dihydrorhodamine 123 (DHR) fluorescent probe.50 51 Briefly, 17 day cultured transduced CD34+ PBHP samples were analyzed on a FACSort (BDIS, San Jose, CA) using Cell Quest software. The photomultiplier tubes for side scatter FL2 (580 nm) were operated using logarithmic amplification. Cells were incubated with DHR at a final concentration of 10 nmol/L for 5 minutes in a 37°C shaking water bath. Samples were then activated by the addition of PMA at a final concentration of 650 nmol/L and then returned to the water bath for an additional 15 minutes. Following incubation, samples were immediately analyzed by flow cytometry. The data was displayed as a dot plot (side scatter on the vertical axis, log flourescence on the horizontal axis) to allow visualization of individual events (cells).
CD34+ PBHP cultured with hematopoietic growth factors were assayed for superoxide production on day 17 using a luminol enhanced chemiluminescence assay of superoxide production as previously described.22 27 This assay was used to assess the output of oxidants by the cell culture population as a whole. Briefly, aliquots of 50,000 cells were placed in 100 μL of Hanks' balanced saline solution in each well of a 96-well flat bottom, white plate (Dynatech Lab, Chantilly, VA). One hundred microliters of Diogenes luminol solution (National Diagnostics, Atlanta, GA) containing 1 μL/mL PMA was added. Superoxide dismutase (Sigma Chemical Co) was used to verify that the luminescence was the result of superoxide generation. Chemiluminescence was read in a Luminoskan luminometer (Lab Products International, Raleigh, NC) in each well for 0.5 seconds, every minute for 40 minutes following stimulation with PMA. Total photon emission (the integral) over this period was taken as the measure of superoxide production. For the cell cultures standard curves were constructed by analyzing different concentrations of cells. In all cases, the curves were linear throughout the range of measurements relevant for these studies.
RESULTS
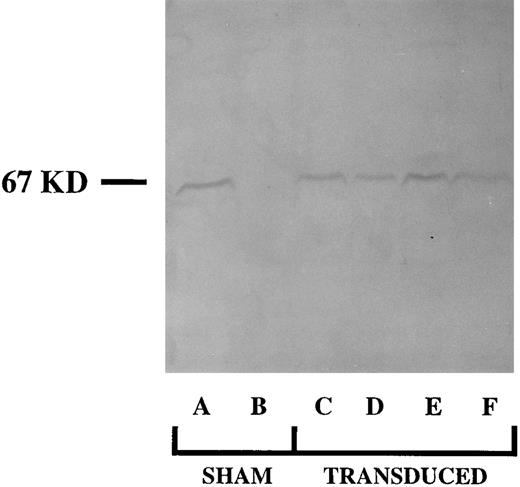
Four MFGS-p67phox retrovirus producer clones were identified, whose supernatant was capable of transducing high levels of expression of p67phox protein in NIH 3T3 fibroblasts. Supernatants from these clones were then used to transduce patient CD34+ cells from a patient with p67phox deficient CGD. Following transduction and growth in culture for 17 days, production of p67phox protein by these differentiating CD34+ cells was confirmed by SDS-PAGE immunoblot (Fig 2). Of note is that the amount of p67phox protein was consistently highest in patient cells transduced with supernatant from clone MFGS-p67phox-20 (Fig 2, lane E). The amount of protein in Fig 2, lane E is similar to the amount of this protein seen with the normal controls with equivalent load of cells per lane. Although these amounts are similar, it is important to note that these samples represent a mixed population of differentiating cells in which the expression of p67phox is constitutive in transduced cells, in contrast to the normal controls, in which expression is restricted to relatively mature phagocytes.
SDS-PAGE immunoblot detection of p67phox protein in 17-day cultures of nontransduced (sham) and transduced CD34+ PBHP. Lane A analyzes a sham treated culture of normal CD34+ cells. Lane B analyzes a culture of p67phox deficient CGD sham treated patient CD34+ cells, lanes C-F analyze cultures of p67phox deficient CGD patient cells transduced with virus derived from MFGS-p67phox clones 2, 5, 20, 37, respectively.
SDS-PAGE immunoblot detection of p67phox protein in 17-day cultures of nontransduced (sham) and transduced CD34+ PBHP. Lane A analyzes a sham treated culture of normal CD34+ cells. Lane B analyzes a culture of p67phox deficient CGD sham treated patient CD34+ cells, lanes C-F analyze cultures of p67phox deficient CGD patient cells transduced with virus derived from MFGS-p67phox clones 2, 5, 20, 37, respectively.
Cultured CD34+ cells underwent differentiation, by day 17 yielding a mixed population of granulocytes and granulocyte precursors as well as monocytes, and earlier progenitors as previously reported.52 The oxidase activity of the maturing cells in this population was determined using the chemiluminescence assay as described in Materials and Methods. PMA was used to maximally activate the NADPH oxidase in all cells capable of response.53 In these studies, cultured CD34+ cells from normal controls were compared to transduced or sham-treated CD34+ cells from a p67phox deficient CGD patient.
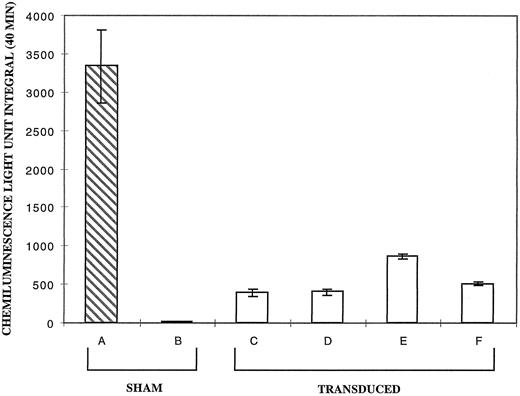
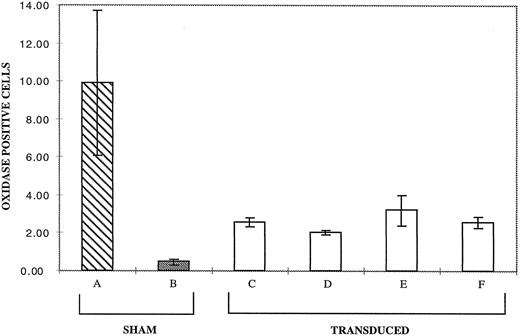
In four separate experiments, supernatants from each of the highest titer clones were used to transduce CD34+ cells from the p67phox deficient CGD patient. Figure 3 shows the results of this analysis expressed as the average light unit integral over 40 minutes. PMA stimulation of the day 17 differentiating sham-treated CD34+ cells from the p67phox deficient patient (Fig 3, bar B) exhibited very low levels of oxidase activity relative to normals (Fig 3, bar A), while cultures of transduced patient cells (Fig 3, bars C through F ) produced significant amounts of superoxide. Supernate from clone 20 (Fig 3, lane E) reproducibly provided the best correction in the different experiments at a mean of 25.3% of the normal. It is of note that clone 20 virus transduction of cells reproducibly resulted in the highest signal on Western Blot (see Fig 2, lane E).
Chemiluminescence assay for superoxide production by PMA activated neutrophils derived from sham treated and transduced CD34+ cells at 17 days of culture. The vertical axis represents the average light unit integral over 40 minutes as a measure of superoxide production. Bar A is the average of six cultures of sham treated normal CD34+ cells. Bars B through F represent the average value of four experiments each with p67phox deficient CGD CD34+ cell cultures for each bar seen. Bar B is sham treated patient cells; bars C through F are patient cells transduced with MFGS-p67phox derived from clones 2, 5, 20, 37, respectively. Error bars represent standard error of the mean.
Chemiluminescence assay for superoxide production by PMA activated neutrophils derived from sham treated and transduced CD34+ cells at 17 days of culture. The vertical axis represents the average light unit integral over 40 minutes as a measure of superoxide production. Bar A is the average of six cultures of sham treated normal CD34+ cells. Bars B through F represent the average value of four experiments each with p67phox deficient CGD CD34+ cell cultures for each bar seen. Bar B is sham treated patient cells; bars C through F are patient cells transduced with MFGS-p67phox derived from clones 2, 5, 20, 37, respectively. Error bars represent standard error of the mean.
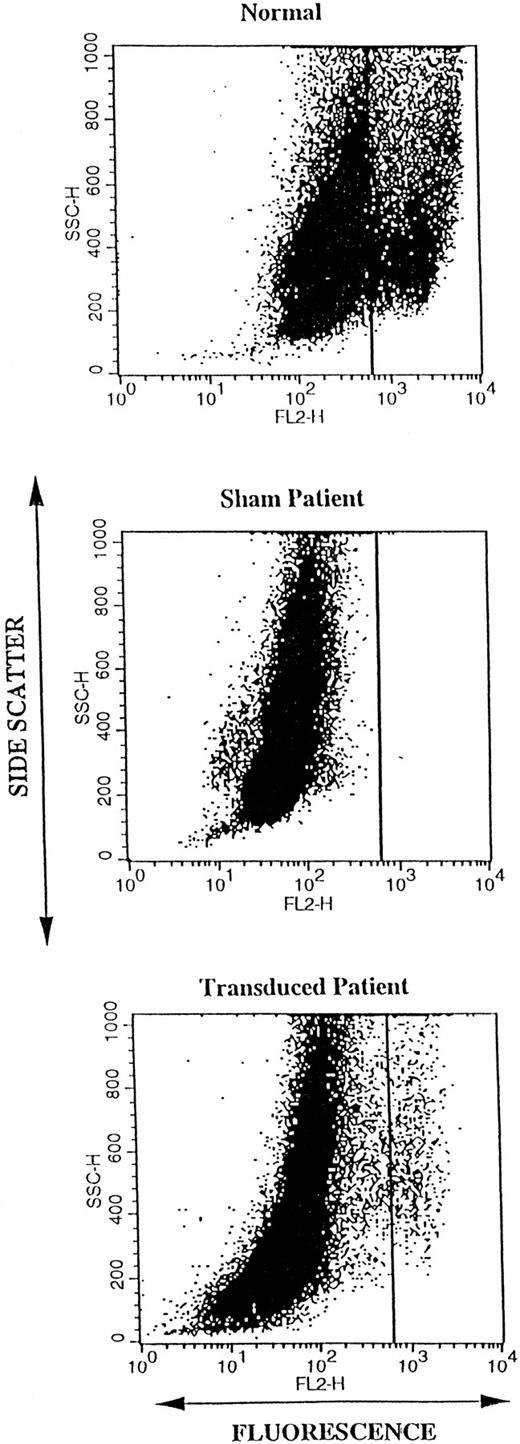
We then used DHR FACS analysis to study the correction of oxidase activity of differentiating transduced p67phox deficient patient CD34+ cells on a per cell basis as opposed to the population based chemiluminescence assay. Cells were again stimulated with PMA as described previously in methods. After 17 days of differentiation in culture, DHR FACS analysis was used to detect the highly oxidase positive granulocytes differentiating in normal cultures and in cultures derived from transduced patient CD34+ cells (Fig 4). Figure 4 is a representative DHR FACS analysis of 17 day differentiating cultures of the normal sham-transduced CD34+ cells (upper panel), patient sham-transduced CD34+ (middle panel), and patient CD34+ cells transduced with MFGS-p67phox clone 20 (lower panel). As seen in the upper panel of Fig 4 only a subset of normal cells (averaging 10%, see Fig 5, bar A) mature to high oxidase activity cells by day 17. In unpublished data, we have found that cells that exhibit high oxidase activity in cultures (the cluster to the right of the vertical line in Fig 4, upper panel) appear to be granulocytes by microscopic observation of sorted cells. In addition, others have shown that oxidase activity is minimal in metamyelocytes and that this activity increases with further cell maturation.53
Dot plot of the DHR FACS oxidase analysis of 17-day cultures of differentiating CD34+ cells. Each dot represents a single “event” (cell) analyzed for side scatter (granularity) plotted in linear scale on the vertical axis, and for fluorescence (oxidant production) plotted in log scale on the horizontal axis. The analysis was gated to include all live cells in the cultures following loading with DHR and activation with PMA. The top panel shows analysis of a typical sham treated normal CD34+ 17-day culture. In this normal CD34+ cell culture, two clusters of cells can be seen that are separated by the vertical line. The large cluster on the left represents immature cells with low-oxidase activity, whereas the smaller cluster to the right represents mature granulocytes (as determined by sorting) with high oxidase activity. The middle and lower panels show analyses of sham treated and MFGS-p67phox transduced p67phox deficient CGD CD34+ cultures, respectively. Mature granulocytes with high oxidase activity are only seen in patient cultures after transduction as in the lower panel.
Dot plot of the DHR FACS oxidase analysis of 17-day cultures of differentiating CD34+ cells. Each dot represents a single “event” (cell) analyzed for side scatter (granularity) plotted in linear scale on the vertical axis, and for fluorescence (oxidant production) plotted in log scale on the horizontal axis. The analysis was gated to include all live cells in the cultures following loading with DHR and activation with PMA. The top panel shows analysis of a typical sham treated normal CD34+ 17-day culture. In this normal CD34+ cell culture, two clusters of cells can be seen that are separated by the vertical line. The large cluster on the left represents immature cells with low-oxidase activity, whereas the smaller cluster to the right represents mature granulocytes (as determined by sorting) with high oxidase activity. The middle and lower panels show analyses of sham treated and MFGS-p67phox transduced p67phox deficient CGD CD34+ cultures, respectively. Mature granulocytes with high oxidase activity are only seen in patient cultures after transduction as in the lower panel.
Results from DHR FACS oxidase analysis of 17-day cultures of differentiating CD34+ cells. Analyses were performed as in Fig 4. The bars represent the percent of cells in the region defined by mature high-oxidase positive granulocytes (those to the right of the line set in Fig 4). Bar A is the average of analysis of seven normals. Bars B through F represent the average value of four experiments for each bar seen. Bar B is sham treated patient cells, bars C through F are patient cells transduced with MFGS-p67phox derived from clones 2, 5, 20, 37, respectively. Error bars represent standard error of the mean.
Results from DHR FACS oxidase analysis of 17-day cultures of differentiating CD34+ cells. Analyses were performed as in Fig 4. The bars represent the percent of cells in the region defined by mature high-oxidase positive granulocytes (those to the right of the line set in Fig 4). Bar A is the average of analysis of seven normals. Bars B through F represent the average value of four experiments for each bar seen. Bar B is sham treated patient cells, bars C through F are patient cells transduced with MFGS-p67phox derived from clones 2, 5, 20, 37, respectively. Error bars represent standard error of the mean.
No high-oxidase positive cells are detected in sham treated 17 day cultures of CD34+ cells from the p67phox deficient patient (Fig 4, middle panel). Figure 4, lower panel illustrates that after transduction, patient cultures contain highly oxidase positive granulocytes as evidenced by a cluster of events in the same region as the high oxidase cells in cultures of normal CD34+ cells. Unlike the sham transduced patient cells, the transduced patient cells are able to produce superoxide through restoration of the NADPH oxidase after transduction with the MFGS-67phox retrovirus supernatant.
Figure 5 expresses the FACS data for four experiments with each group expressed as a percent of the cells located in the region to the right of the line shown in Fig 4, which we have determined to be occupied by high-oxidase positive cells. Again, producer clone 20 yielded MFGS-p67phox virus capable of transducing the greatest number of cells. As indicated in Fig 5, lane E, a mean of 3.2% of cells are high oxidase positive compared to 10% in the normal cell cultures (Fig 5, lane A). Thus, using normal cells as a reference, this suggests a transduction correction rate of 32%. High-oxidase positive cells in patient CD34+ cell cultures transduced with clone 20 had an average fluorescence channel intensity of 896.5 ± 304.99 over four experiments. In six different normal CD34+ cell cultures the high-oxidase positive cells exhibited a mean fluorescence of 1083.79 ± 591.56. As illustrated in Fig 4, the mean fluorescence of high-oxidase positive cells in transduced patient cell cultures was slightly less than normal with a mean of 85% of the fluorescence of normal high-oxidase positive cells in CD34+ cell cultures. It is not known if this difference in mean fluorescence found in the DHR FACS analysis corresponds linearly to the difference in actual superoxide production, but does suggest a quantitative difference.
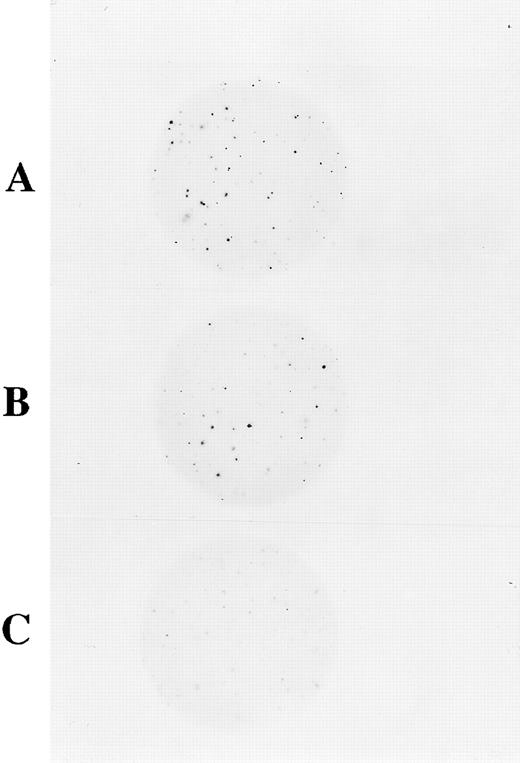
Superoxide reduces NBT to a blue-black formazan precipitate. Cells capable of producing superoxide upon activation with PMA will stain blue-black due to reduction of NBT. We assayed myeloid colonies derived from CD34+ cells using NBT and PMA activation in agarose colony cultures described in the methods. Scoring was separately determined for the GEM mixed colonies derived from multipotential progenitor cells and for the group of granulocyte, monocyte, or granulocyte-monocyte mixed (G/M/GM) colonies derived from more committed progenitor cells. Figure 6 shows at low magnification the NBT staining of colonies in agarose. When 3,000 CD34+ cells were plated, normal PBHPs cultured in semisolid agarose for 14 days formed 66 GEM colonies of which 61 were strongly NBT positive. In the same culture, 193 colonies were G/M/GM colonies of which 178 were strongly NBT positive colonies. In the same assay, transduction of patient CD34+ PBHPs with supernatant from clone MFGS-p67phox -20 resulted in the appearance of 25 out of 57 (44%) strongly NBT positive GEM colonies and 98 out of 346 (28%) strongly NBT positive in the group G/M/GM colonies. As can be seen in Fig 6, similar sham transduced patient p67phox-CGD PBHPs formed no strongly NBT positive colonies. When scored at high magnification the sham treated patient cell cultures were as follows: 0 strongly NBT positive out of 143 GEM colonies and 0 strongly NBT positive out of 322 in the group of G/M/GM colonies. For most colonies derived from a single progenitor cell, all maturing myeloid cells in a single colony were either NBT positive or negative.
NBT-dye reduction of PBHP myeloid colonies in agarose culture. These are photomicrographs at low magnification (2× final magnification) where each dark spot represents a colony, containing strongly oxidase positive cells that have reduced NBT to formazan. (A) Colonies derived from normal CD34+ progenitor cells showing positive NBT-dye reduction to blue-black formazan. (B) Colonies derived from p67phox deficient CGD patient CD34+ cells transduced with MFGS-p67phox clone 20 exhibiting strong NBT-dye reduction of many colonies. (C) Colonies derived from sham transduced patient CD34+ cells, which exhibit only weak background NBT-dye reduction.
NBT-dye reduction of PBHP myeloid colonies in agarose culture. These are photomicrographs at low magnification (2× final magnification) where each dark spot represents a colony, containing strongly oxidase positive cells that have reduced NBT to formazan. (A) Colonies derived from normal CD34+ progenitor cells showing positive NBT-dye reduction to blue-black formazan. (B) Colonies derived from p67phox deficient CGD patient CD34+ cells transduced with MFGS-p67phox clone 20 exhibiting strong NBT-dye reduction of many colonies. (C) Colonies derived from sham transduced patient CD34+ cells, which exhibit only weak background NBT-dye reduction.
DISCUSSION
In the present studies we used the MFGS-p67phox retrovirus encoding the oxidase protein p67phox to genetically correct the CGD phenotype of myeloid cells derived from transduced CD34+ PBHP from a p67phox-deficient CGD patient. Recent studies in the clinical setting of postchemotherapy autologous transplantation suggests that CD34+ PBHP can substitute for BM in autologous transplants leading to permanent reconstitution of hematopoietic function,54-57 indicating that the earliest stem cells are represented in the PBHP population. However, in our studies it is not possible to know to what extent we are transducing totipotent stem cells. In addition, culturing of progenitor cells necessary for optimum transduction could lead to differentiation of the earliest progenitors and loss of reconstitution potential. We also did not examine transduction of long-term culture initiating cells (LTC-ICs), but we do assess transduction of GEM colonies, and in addition our liquid cultures in some cases were maintained as long as 30 days, with detection of continuous production of oxidase corrected granulocytes by transduced CGD progenitors. The appearance of strongly NBT-positive large mixed lineage colonies in the agarose cultures indicate that at least some of the transduced CD34+ cells are early multipotential progenitors. Moreover, with most strongly NBT positive colonies, all maturing granulocyte-monocyte cells are NBT positive indicating stability of expression of the transduced oxidase gene. Also, the more than 80-fold increase in cell number in liquid cultures, which was similar in the sham and transduced samples suggests that transduction did not adversely affect proliferation. Furthermore, the percent of erythroid colonies in sham and transduced agarose colony cultures was similar indicating no effect on erythroid differentiation.
In the liquid cultures, the high-oxidase positive cells derived from transduced CD34+ CGD progenitors were able to produce total amounts of superoxide upon PMA stimulation that appeared to approach normal levels. We used a variety of assays to measure superoxide production as we feel that each assay quantitates superoxide production in a slightly different manner. DHR FACS analysis was used to quantitate both the number of high-oxidase positive granulocytes (32% of normal) and their average fluorescence intensity (85% of normal). Chemiluminescence assay was used to determine the total oxidant production of a given culture. This assay suggested a value of 25% of normal with PMA stimulation. The results of the DHR FACS and the chemiluminescence assay appear to be in accordance when one accounts for the average fluorescence intensity of 85% of normal for the transduced cells in the DHR FACS analysis.
As indicated above, MFGS-p67phox contained no selectable marker, and the degree of functional correction of PBHP seen in these studies occurred without selection to enrich for transduced cells. This mimics the procedure used in the initial clinical phase I trial of gene therapy for the p47phox deficient form of CGD, whereby CD34+ PBHP were transduced in vitro and then reinfused back into patients without selection.47 Eventually, it may prove practical in a next generation of vectors to incorporate some type of resistance gene that is selectable in vivo, such as multiple drug resistance-1 to provide a selective growth advantage following transfusion with gene corrected progenitors.58
Since correction of oxidase activity provides no known growth advantage to transduced CGD CD34+ progenitors, achieving high rates of transduction is an important goal. The almost 32% oxidase function corrected granulocytes in culture achieved with supernatant from producer clone MFGS-p67phox-20 is considerably higher than previously reported rates for functional correction of the granulocytes derived from transduced CGD CD34+ progenitors, representing an important improvement.22,27 Similar rates of correction are now being achieved in our laboratory47 with higher titer MFGS-p47phox, MFGS-p22phox, and MFGS-gp91phox vectors, suggesting that improved virus titers, together with modification of growth and transduction conditions used for the present study may play a role in this improvement.
As we have previously suggested, it may not be necessary to achieve high levels of engraftment of corrected cells to provide some benefit to CGD patients, though high levels of correction within individual neutrophils likely are important. When small numbers of normal neutrophils are mixed with a much larger number of CGD neutrophils in aspergillus killing assays in vitro, there is synergistic enhancement of the killing activity compared to including only the normal cells in the assay.59 This suggests that normal cells produce sufficient excess hydrogen peroxide to diffuse into CGD neutrophils to “arm” the CGD neutrophils by interacting with the nonoxidative killing mechanisms still present in CGD neutrophils.60 Finally, female carriers of the X-linked form of CGD are mosaics for the CGD neutrophil phenotype.61 Recently, we have found a number of such X-linked CGD carrier females with less than 5% normal oxidase positive neutrophils. One such adult mother of an X-linked CGD patient had only 3% normal cells, yet had no evidence of increased susceptibility to infection. These mosaics exhibit full oxidase activity in their functioning neutrophils whereas we have found that genetically corrected p67phox patient CD34+ cells differentiated to neutrophils in culture achieved 85% of normal fluorescence activity as measured by DHR FACS analysis. This may be sufficient to achieve the synergistic effect on microbial killing required for clinical benefit.
ACKNOWLEDGMENT
The authors thank Dr John Gallin and all the members of the Laboratory of Host Defenses for their advice and keen scientific insights.
Address reprint requests to Harry L. Malech, MD, Laboratory of Host Defenses, National Institute of Allergy and Infectious Diseases, Bldg 10, Room 11N 113, National Institutes of Health, Bethesda, MD 20892-1886.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal