Abstract
We report the development of an enzyme-linked immunosorbent assay (ELISA) that is specific for factor VIIa (FVIIa). This assay uses a neoantigen specific capture antibody directed to the amino acid peptide sequence N terminal to the FVII cleavage activation site. The antibody exhibits ∼3,000-fold greater reactivity to FVIIa than FVII on a molar basis. Experiments using plasma with added (exogenous) human FVIIa gave quantitative recovery in the ELISA over a range of 0.20 to 3.2 ng/mL of FVIIa. The intra- and inter-assay coefficient of variation (CVs) of the ELISA are 4.5% and 9.8%, respectively. The ELISA shows excellent correlation (r = .99) with a functional assay (using recombinant soluble tissue factor) in detecting FVIIa added to plasma over the range 0.05 to 18.0 ng/mL. However, a major discrepancy exists between the two assays when normal endogenous plasma concentrations of FVIIa are measured. Using normal plasma (n = 14) the functional assay reported 3.10 ± 0.30 ng/mL (mean ± SE) whereas only 0.025 ± 0.010 ng/mL was detected in the same samples by the immunoassay. Patients (n = 43) presenting with acute coronary syndromes (myocardial infarction and unstable angina) exhibited elevations (P < .05) in immunologically detected FVIIa, 0.093 ± 0.013 ng/mL (mean ± SE) compared to patient controls (n = 20) contemporaneously admitted with noncardiac chest pain, 0.048 ± 0.007 ng/mL (mean ± SE). These elevations in the acute coronary syndromes were accompanied by increased (P < .05) and correlating prothrombin fragment F1 + 2 levels (Spearman correlation coefficient rs = .4, P < .01), demonstrating that thrombin generation is certainly associated with, and may even be caused by, extrinsic pathway activation.
FACTOR VII (FVII) is the first zymogen of the extrinsic pathway of blood coagulation. Factor VIIa (FVIIa) is generated by cleavage of FVII at Arg152-Ile153 by proteinases such as factor Xa (FXa), giving rise to a light and a heavy polypeptide chain held together by a disulfide bond between residues Cys135 and Cys262. When FVIIa is bound to tissue factor it forms an activating complex of potential importance in hemostasis and thrombosis. Regulation of the activity of FVIIa is thought to be mediated by tissue factor pathway inhibitor (TFPI).1
Determination of FVIIa levels in plasma have long been of interest but there has been uncertainty over the optimum means of measurement.2,3 Some studies using methods with uncertain specificity have attempted to differentiate between FVIIa and FVII as risk factors for cardiovascular disease. Conflicting conclusions have been drawn, with either factor claimed to be important.4-8 A recent collaborative investigation of existing FVII assays has concluded that assays which reflect both zymogen and FVIIa may be preferable for estimating risk of acute coronary events.9 Direct and specific determination of FVIIa is required to clarify its role in ischemic heart disease and other clinical situations characterized by provoked coagulation activation.
Neuenschwander and Morrissey10 reported that truncated soluble tissue factor (residues 1-219) is able to bind FVIIa, forming an effective activator of factor X. A consequence of truncation of tissue factor is that activation of FVII is greatly reduced by the truncated tissue factor-FVIIa complex. These observations provided the basis for development of a direct functional assay to measure FVIIa and the principle of selective activity of FVIIa with truncated tissue factor underlies the existing specific functional clotting assays11,12 and a fluorogenic assay.8 Although truncated tissue factor is undoubtedly more selective in forming an activating complex with FVIIa only (in plasma), the assay specificity may not be absolute, as it has been shown that tissue factor-independent activation of FVII by FXa in the presence of truncated tissue factor can occur.10 Activation of FVII by truncated tissue factor-FVIIa complex can also occur, depending on the functional assay conditions.13 Should even a small proportion of FVII be activated during the course of the functional assay, specificity would be compromised, in view of the great molar excess of the zymogen.
We describe here the development of an alternative means of determination of FVIIa levels that does not depend on its functional activity. We have developed an enzyme-linked immunoabsorbent assay (ELISA) to determine free FVIIa antigen in plasma. This uses a unique capture antibody with a high specificity toward two-chain FVIIa. We report a comparison of this assay with the functional assay, the determination of plasma levels of two-chain FVIIa in normal subjects and in patients with acute coronary syndromes (myocardial infarction [MI] and unstable angina).
MATERIALS, METHODS, AND PATIENTS
Materials
Recombinant FVIIa was purchased from NovoNordisk (Crawley, UK). Human FVII, human FVIIa, and a polyclonal antibody to FVII labeled with horseradish peroxidase were purchased from Enzyme Research Laboratories (South Bend, IN). Human FVIIa was also prepared by activating FVII using FXa from Chromogenix (Epsom, UK). FVII deficient plasma was from Diagen (Oxon, UK). Truncated soluble tissue factor (TF220 ) was a kind gift from Prof E.G. Tuddenham (CSC, Hammersmith Hospital, London, UK). Rabbit brain cephalin was purchased from Sigma (Poole, UK). Maleimide activated carriers (bovine serum albumin [BSA] and keyhole limpet hemocyanin [KLH]), gel filtration columns, and Ellman's reagent were from Pierce (Rockford, IL). AMPAK, an alkaline phosphatase enzyme amplification kit, was purchased from Dakopatts (High Wycombe, UK). Hammerstan casein was purchased from BDH (Poole, UK). Flat-bottomed Maxisorb microtiter plates and strip-wells were from Nunc (Kamsrtup, Denmark). Synthetic peptides were purchased from the Advanced Biotechnology Centre, Charing Cross Hospital, London, UK. These were synthesized on an Applied Biosystems (Cheshire, UK) Peptide Synthesizer (model 431A) using f-moc chemistry performed by the solid-phase method of Merrifield.14 Monoclonal antibody to FVII, F8146, was from Sigma. A polyclonal antibody to FVII was a kind gift from Dr G. Kemball-Cook (CSC, Hammersmith Hospital). Plasma from a patient with hemophilia A infused with recombinant FVIIa was supplied by Prof P.M. Mannucci (Milan, Italy). EA anticoagulant was prepared as described previously15 and comprised 0.22% (wt/vol) EDTA, 2.2% (wt/vol) trisodium citrate, 0.73% (wt/vol) citric acid, 25 U/mL hirudin (Ciba Geigy Pharmaceuticals, Horsham, UK), 0.16% (wt/vol) adenosine, 2.45% (wt/vol) dextrose. Reptilase was purchased from Diagnostica Stago (Asnieres, France). Biotin-XX-NHS was from Calbiochem (San Diego, CA). An FPLC column of NHS-activated Superose was purchased from Pharmacia (Milton Keynes, Bucks, UK).
Methods
Plasma preparation.Blood was collected from normal laboratory controls and from patients into 0.105 mol/L sodium citrate (Becton Dickinson, Meylan Cedex, France). The ratio of blood to anticoagulant was 9:1 (vol/vol). Platelet-poor plasma obtained by centrifugation at room temperature for 10 minutes at 2,000g, was then snap-frozen on dry ice.
Preparation of neoantigen-specific (capture) antibody to FVIIa.The first step in the development of the ELISA for FVIIa was to raise an antibody to a region of FVII exposed following its activation by cleavage at Arg152-Ile153.16 The candidate polypeptide sequences were the newly created termini of FVIIa that become exposed after cleavage activation. The new C-terminal amino acid sequence of the FVIIa light chain ending at Arg152 possesses more hydrophilic residues than the new N-terminal amino acid sequence of the heavy chain beginning at Ile153. The former was chosen as an immunogen because solubility was required for coupling to the carrier molecule and hydrophilic peptides are generally more antigenic. A synthetic peptide with the primary amino acid sequence of this region was prepared, with the addition of a cysteine residue to allow heterobifunctional conjugation to a carrier protein. The sequence of this ondecapeptide was NH2 -Cys-Glu-Lys-Arg-Asn-Ala-Ser-Lys-Pro-Gln-Gly-Arg-CO2H.
One volume of sulfydryl-containing peptide was reconstituted at 10 mg/mL in conjugation buffer (0.083 mol/L sodium phosphate, 0.15 mol/L NaCl, 0.1 mol/L EDTA, 0.02% [wt/vol] sodium azide pH 7.2). The preactivated carrier proteins, BSA and KLH, were dissolved in distilled water at 10 mg/mL. One volume of preactivated carrier protein was mixed with 1 vol of peptide and incubated at room temperature for 2 hours. The conjugate was purified by passage through a gel filtration column (exclusion limit 5,000 MW). A qualitative estimate of the success of coupling was made using Perkin Elmer (Warrington, UK) capillary electrophoresis equipment (performed by the Advanced Biotechnology Centre, Charing Cross Hospital). Samples for analysis were taken from the peptide solution before conjugation and from the conjugate mixture after the reaction. An additional estimate of conjugation following coupling via sulfo-SMCC groups was made by measuring the number of sulfdryl groups present before and after the coupling reaction. This was done using Ellman's reagent [5,5′-dithiobis-(2-nitrobenzoic acid)], which reacts with sulfydryls to form a highly colored chromophore having an absorbance maximum at 412 nm (E412nm = 1.36 × 104 cm−1M−1). Samples were monitored following a 15-minute incubation with 1 mg/mL of Ellman's reagent.
New Zealand White rabbits were inoculated subcutaneously with 0.3 mg of peptide-carrier protein conjugate mixed with Freund's Complete Adjuvant. The second immunization was given after 4 weeks; the same amount of conjugate was administered, mixed with Freund's Incomplete Adjuvant. Thereafter, 6-week intervals were allowed between immunizations where one quarter of the initial amount of conjugate was administered, diluted in physiological saline without adjuvant. Blood (∼30 mL) was collected by ear venepuncture 10 days after receiving the second and subsequent immunisations and antisera were prepared by standard methods.
Peptides were coupled directly to the NHS-activated Superose column essentially according to the manufacturer's instructions. The ligand solution was injected and left stationary for 1 hour before being washed away. Affinity chromatography using the immobilized peptide column with FPLC equipment, was used to isolate anti-peptide antibodies from antisera.
Biotin labeling of antibodies.The antibodies (1 mg/mL) were dialyzed for 6 hours in 200 vol of 10 mmol/L azide-free phosphate-buffered saline (PBS) (with two changes of solution), followed by dialysis in 400 vol of 0.1 mol/L Na2CO3 , pH 8.5, for 16 hours. Biotin-XX-NHS was dissolved in dimethylformamide and the appropriate volume was added to the antibody population to yield a molar ratio of biotin-XX-NHS to IgG of 75. The solution was incubated for 60 minutes at room temperature while mixing on a roller mixer. The reaction was stopped by the addition of 20 μmol of NH4Cl (per mg of IgG) incubated for 10 minutes at room temperature. Labeled antibody was dialysed against 200 vol of 10 mmol/L PBS with 0.02% (wt/vol) sodium azide (NaN3 ), with two changes of solution over 24 hours and subsequently dialysed against 200 vol of 10 mmol/L PBS with 0.12% (wt/vol) NaN3 for 16 hours to yield a final concentration of 0.1% (wt/vol) NaN3 . The final preparation was centrifuged at 2,000g and the supernatant was removed as clarified biotin-labeled antibody.
ELISA for FVIIa.Wells of a Maxisorb plate were incubated with 100 μL of 10 μg/mL of cleavage neoantigen specific capture antibody to FVIIa in 100 mmol/L Na2CO3 , pH 9.6, for 16 hours at 4°C. The wells were washed between incubations with 10 mmol/L sodium phosphate, 500 mmol/L NaCl, 0.1% (vol/vol) Tween-20, pH 7.4 (high-salt PBS-Tween), and then blocked using 315 μL of 10 mmol/L PBS containing 1% (wt/vol) casein (PBS/casein), incubated at 37°C for 6 hours. While there is a standard for FVIIa available from the National Institute for Biological Standards and Control, Herts, UK, this material is calibrated only in terms of activity, and not weight. The recombinant FVIIa purchased from NovoNordisk was therefore used directly as a reference material. The amount of FVIIa supplied by the manufacturer was determined by amino acid composition. Working standards (0 to 7.80 ng/mL of recombinant FVIIa diluted in PBS/casein), controls, and samples, were diluted twofold in high-salt PBS-Tween. One hundred microliters of the diluted antigen was incubated in each well for 1 hour at 37°C and this was followed by incubation with 100 μL of an appropriate dilution of biotin-labeled monoclonal antibody to FVII, F8146, or polyclonal antibody to FVII in PBS/casein-0.05% (vol/vol) Tween. This was followed by incubation with 100 μL of an appropriate dilution of streptavidin-alkaline phosphatase diluted in PBS/casein-Tween. The wells were then washed manually using the AMPAK wash buffer diluted 40-fold. One hundred microliters of the AMPAK substrate supplemented with 1 mmol/L MgCl2 was added to the wells of the plate incubated at room temperature for 20 minutes in the dark. Without washing the substrate from the wells, 100 μL of AMPAK amplifier solution was added to each well and incubated for 10 minutes at room temperature in the dark. The reaction was stopped by the addition of 50 μL stop reagent and plates were then read at 492 nm using an iEMS plate reader MF (Labsystems, Basingstoke, UK).
Functional assay for FVIIa.The protocol used was a slight modification of that used by Morrissey et al.12 Soluble tissue factor220 (TF220 ) was used at a concentration of 32 μg/mL diluted in 100 mmol/L NaCl, 50 mmol/L Tris.HCl, pH 7.4 containing 0.02% NaN3 , 0.1% (wt/vol) BSA (TBS/BSA). Samples for a seven-point standard curve ranging from 0.05 to 216 ng/mL of recombinant FVIIa were diluted in FVII-deficient plasma (Diagen, Oxon, UK), and subsequently diluted again twofold in FVII-deficient plasma. Samples were initially diluted 1 in 5 in FVII-deficient plasma and subsequently further diluted twofold in FVII-deficient plasma. Rabbit brain cephalin was diluted with 0.85% saline to yield double the concentration recommended by the manufacturer, diluted 1 in 2 with diluted TF220 .
Clotting times were measured using an ACL 300 Research coagulometer (Instrumentation Laboratory, Milan, Italy) operated in research mode. The settings were 64 seconds activation time, 1 second inter-ramp interval, 0 seconds delay, 250 seconds acquisition time, 1,200 rotor speed. Fifty microliters of sample was aspirated, followed by 100 μL of platelet substitute/TF220 . The clotting time was determined after addition of 50 μL of 0.025 mol/L CaCl2 . A standard curve reporting concentrations in nanograms per milliliter of FVIIa was generated using the Genesis software package (LabSystems, Basingstoke, UK).
Western blotting.Proteins to be targeted were electrophoresed using Novex (San Diego, CA) precast gels. Samples were diluted 1 in 2 with sodium dodecyl sulfate (SDS) sample buffer and run at a constant voltage of 125 V in SDS-polyacrylamide gel electrophoresis (SDS-PAGE) running buffer. When the marker dye had run to the end, the gel was placed in 100 mL of transfer buffer to equilibrate. The gel was arranged adjacent to the cathode electrode and the nitrocellulose membrane next to the gel closer to anode, in the transfer tank. Blotting took place with current at 0.5 A for 90 minutes, in the presence of ice to prevent overheating. The nitrocellulose membrane was removed and incubated in NET buffer (0.15 mol/L NaCl, 0.05 mol/L Tris-HCl, 0.05% [vol/vol] Triton X-100, 0.25% [wt/vol] gelatin, pH 7.4) for 1 hour at room temperature to block any nonspecific binding sites, followed by incubation with approximately 10 μg of primary antibody towards FVII, in NET buffer for 1 hour at room temperature. The membrane was washed three times with 10-minute incubations in NET buffer between incubations, followed by a 1-hour incubation with the secondary antibody directed against the species of the primary antibody conjugated to horseradish peroxidase. After washing, ECL detection reagents 1 and 2 were mixed in equal volume quantities with the addition of approximately 5 μL of 30% (vol/vol) hydrogen peroxide for each 2 mL of combined reagents. The membrane was placed in a detection folder and the combined detection reagent was poured over the membrane which was then exposed to Kodak X-OMAT autoradiographic film (Eastman Kodak, Rochester, NY) for approximately 1 minute.
Patient samples.Samples were collected for FVIIa and F1 + 2 analysis from patients presenting at Charing Cross Hospital Accident and Emergency Department who were subsequently admitted to the Coronary Care Unit with chest pain and suspected acute coronary syndromes. In the absence of a diagnostic ECG, rest pain was an obligatory criterion for admission, thereby excluding patients with stable angina. The same sampling officer performed the venepuncture on patients admitted to the Department for investigation of chest pain. Samples were collected prospectively over a 6-month period. Verbal consent from patients was obtained before sampling (approved by local Ethics Committee). All patients were sampled before treatment with IV heparin or thrombolysis. Venous blood was collected from a cuffed ante-cubital upper limb vein. Plasma was prepared as described above and stored at −70°C within 70 minutes of obtaining the samples. Patients were subsequently retrospectively classified to a final diagnostic group shown in Table 1 once all investigations were complete. Patients taking oral anticoagulants on admission were excluded from analysis.
Myocardial infarction was diagnosed according to the World Health Organization (WHO) criteria (Working Group on the Establishment of Ischaemic Heart Disease Registers: Report of the Fifth Working Group, WHO, Eur 8201 [5], Copenhagen, Denmark, 1971). Unstable angina was diagnosed in the absence of myocardial infarction [all cardiac enzyme measurements (creatine kinase, aspartate transaminase, hydroxybutyrate dehydrogenase) were below twice the upper reference range throughout the routine sampling period (daily for 3 days)] from evidence of ischemic heart disease demonstrated by either follow up cardiac event (death, coronary revascularisation, or myocardial infarction), a positive exercise treadmill test (>0.1 mV ST segment depression 80 milliseconds after the J point), or demonstration of ischemia on thallium radio-isotopic scanning. Noncardiac chest pain was diagnosed if angina was excluded by an alternative source of pain, or a negative exercise treadmill test, or normal coronary anatomy on subsequent angiography. Final diagnosis in patients who were thought not to have had acute coronary syndromes (patients with noncardiac chest pain) were pneumonia (n = 1), gastrointestinal origin (n = 6), musculoskeletal (n = 7), and nonspecific origin (n = 6).
Statistical analysis.In the development stage of the assay where paired analysis of data was required, the Student paired t-test was used. Linear regression analysis was used to compare the results of ELISA and functional assays for FVIIa. Comparison of activation marker levels between patient groups was performed using the Wilcoxon-Mann-Whitney test. Spearman correlation analysis was used to investigate the relationship of FVIIa antigen with F1 + 2 levels in patients with acute coronary syndromes.
RESULTS
Development of FVIIa ELISA
The specificity of the capture antibody directed to FVIIa was determined initially using the peroxidase-labeled polyclonal antibody directed to FVII (because ultra sensitivity was not required for these particular experiments, there was no need to incorporate the additional AMPAK amplification of signal) as a tag antibody (see Fig 1A). Capture antibody obtained from different rabbits showed variable selectivity for FVIIa (recombinant or human) relative to FVII ranging between 1,300- and 3,300-fold on a molar basis, one with the latter extreme being selected for use. These results provide minimum estimates as it is likely that the FVII preparation would have contained trace amounts of FVIIa. Specificity was further explored by Western blotting, using a gel loaded with 1 μg/mL of FVII (twice the normal physiological concentration), adjacent to FVIIa. The results illustrated in Fig 1B show no recognition of FVII, but its recognition after activation and equivalent recognition of recombinant FVIIa. This confirmed that the specificity of the antibody toward FVIIa was sufficient to prevent interference by its zymogen at physiologic concentrations. These data suggested the potential suitability of this antibody for development of a specific ELISA for FVIIa.
(A) Specificity of FVIIa neoantigen specific capture antibody. Human FVII (ERL) (⋄), human FVII (ERL) activated with commercial factor Xa (□), and recombinant FVIIa (NovoNordisk) (•). The ELISA was performed using a tag antibody directed to FVII labeled with peroxidase (ERL), as amplification of the FVIIa signal was not necessary. (B) Western blot analysis of FVIIa neoantigen specific capture antibody. Lane A, human FVII (ERL); lane B, recombinant FVIIa (NovoNordisk); lane C, human FVIIa (ERL); lane D, human FVII (ERL) activated by factor Xa. Human FVIIa from ERL is des-Gla and has a reduced molecular weight. Initial electrophoresis was performed with samples that were not reduced (Non-Reducing) and that were reduced with dithiothreitol.
(A) Specificity of FVIIa neoantigen specific capture antibody. Human FVII (ERL) (⋄), human FVII (ERL) activated with commercial factor Xa (□), and recombinant FVIIa (NovoNordisk) (•). The ELISA was performed using a tag antibody directed to FVII labeled with peroxidase (ERL), as amplification of the FVIIa signal was not necessary. (B) Western blot analysis of FVIIa neoantigen specific capture antibody. Lane A, human FVII (ERL); lane B, recombinant FVIIa (NovoNordisk); lane C, human FVIIa (ERL); lane D, human FVII (ERL) activated by factor Xa. Human FVIIa from ERL is des-Gla and has a reduced molecular weight. Initial electrophoresis was performed with samples that were not reduced (Non-Reducing) and that were reduced with dithiothreitol.
An ELISA for FVIIa was developed using the capture antibody and two alternative tag antibodies; polyclonal antibody raised to human purified FVII (from Dr G. Kemball-Cook) and commercial monoclonal antibody to FVII, F8146. The two tag antibodies were equally effective at reporting FVIIa capture signal. (Although the polyclonal antibody was finally adopted for routine investigation of plasma samples as the slope sensitivity of the standard curve was somewhat greater, some results obtained in the development stage of the assay using the monoclonal antibody as tag will be presented.) Optimal slope sensitivity for FVIIa was achieved using 10 μg/mL of the capture antibody, a 1/1,000 dilution of the polyclonal tag antibody, and a 1/4,000 dilution of the streptavidin-phosphatase conjugate.
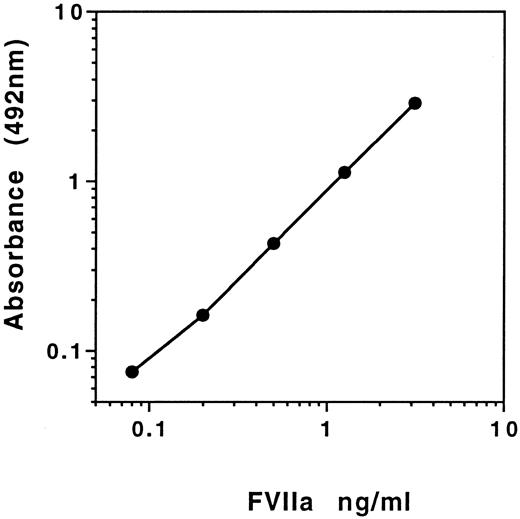
A typical standard curve obtained for the ELISA is presented in Fig 2. Using serial dilution of FVIIa toward the blank, concentrations of 0.01 ng/mL FVIIa could be estimated. Such detectability was made possible using the amplification (AMPAK) stage in the assay.
Standard curve of the FVIIa ELISA prepared using recombinant FVIIa (NovoNordisk) as standards and using Ampak amplification of signal.
Standard curve of the FVIIa ELISA prepared using recombinant FVIIa (NovoNordisk) as standards and using Ampak amplification of signal.
Recovery of FVIIa added to normal plasma calibrated against FVII-deficient plasma was determined using human FVIIa and recombinant FVIIa. The results are illustrated in Table 2. Differences in the recovery of recombinant FVIIa were observed at very low and high concentrations between the ELISAs and functional assays. The recovery of recombinant FVIIa was approximately 75% to 151% over the range of 0.20 to 12.8 ng/mL using the ELISA. With the functional assay the recovery of recombinant FVIIa was around 83% to 193% over the same range (Table 2). Recovery experiments performed using human FVIIa in the ELISA were more constant (90% to 120%) over the range investigated (0.20 to 3.20 ng/mL) (Table 2). Experiments to evaluate recovery of human and recombinant FVIIa from FVII-deficient plasma calibrating against buffer gave essentially identically results in the ELISA. As the functional activity assay may only be calibrated using FVII-deficient plasma, recovery of FVIIa from FVII-depleted plasma (using a calibration curve performed with buffer) could not be performed.
As a means of investigating the linearity of the assay, a plasma sample from a patient infused with recombinant FVIIa (ie, with a high FVIIa concentration), was diluted over a range of 3- to 27-fold in buffer (10 mmol/L PBS/1% casein) and in FVII-deficient plasma. The results in Table 3 suggest good linearity over the given range of dilutions.
ELISA precision was monitored using normal plasma spiked with 3.0 ng/mL of FVIIa. The intra-assay CV was 4.5% (n = 12) and the inter-assay CV was 9.8% (n = 7).
Comparison of ELISA With the Functional FVIIa Assay
A single normal plasma sample to which increasing amounts (in the range of 0.05 to 18.0 ng/mL) of recombinant FVIIa was added was assayed using the functional assay and the FVIIa ELISA. The results illustrated in Fig 3 show that both assays have an excellent linear response to added FVIIa. The correlation between results of the functional assay and the ELISA was excellent, r = .99 (P < .01). However, there is a major discrepancy between the functional assay and the immunoassay regarding results of baseline concentrations (ie, without added FVIIa) in this normal plasma, 2.64 compared with 0.15 ng/mL, respectively.
Comparison of the functional activity assay (using truncated tissue factor) and the ELISA for FVIIa. To a single normal plasma was added increasing amounts of recombinant FVIIa and the FVIIa level in plasma was determined experimentally.
Comparison of the functional activity assay (using truncated tissue factor) and the ELISA for FVIIa. To a single normal plasma was added increasing amounts of recombinant FVIIa and the FVIIa level in plasma was determined experimentally.
Determination of Normal Plasma Levels of FVIIa
Plasma taken from normal laboratory staff (n = 14), mean age 35, range 21 to 51 years, was assayed for FVIIa levels using both the functional assay and the ELISA. Using the functional assay a result of 3.10 ± 0.29 ng/mL (mean ±SE) was determined, whereas the immunoassay gave a result of 0.025 ± 0.010 ng/mL.
To ensure that binding of FVIIa to lipid in plasma was not preventing its immunologic recognition, normal plasma from the same subjects were assayed for FVIIa with and without incubation with Triton-X-100. The concentration of this detergent used (0.5% [vol/vol]) was sufficient to enable extraction of proteins from miscelles.19 Addition of Triton-X-100 to standards did not alter the standard curve. Plasma samples gave very similar values whether or not they were incubated with detergent (0.038 ± 0.015 ng/mL and 0.062 ± 0.025 ng/mL, mean ±SE, n = 9, not significant [NS], respectively).
Trace amounts of tissue factor may circulate in plasma and if complexed to FVIIa might impair its immunologic recognition. The effect of binding of tissue factor to FVIIa was investigated by adding TF220 at a concentration of 580 ng/mL to increasing concentrations of FVIIa (0.20 to 7.80 ng/mL in PBS/casein) in the presence of calcium. No change in concentration of FVIIa was reported by the ELISA, using either the monoclonal or polyclonal tag antibodies. This was also observed when TF220 was added to normal plasma samples (without added calcium ions) (0.031 ± 0.19 ng/mL and 0.035 ± 0.019 ng/mL, mean ±SE, n = 6, NS).
Activation of FVII During the Functional Assay
Activation of FVII during the FVIIa functional assay could explain the discrepancy between normal FVIIa levels determined using the functional assay and the ELISA. To examine this, FVIIa immunologic levels were determined on the products of a functional assay, modified to prevent clot formation. Fibrinogen was initially removed from plasma using Reptilase and the procedures of the functional assay were then performed manually. The functional assay had to be modified by not diluting in FVII-deficient plasma in order that sample dilution did not reduce FVIIa levels below the limit of detection of the ELISA. Accordingly, smaller volumes of components (TF220 , rabbit brain cephalin, and CaCl2 ) at higher concentrations were used, while keeping their final concentrations the same as the conventionally performed assay. The reactions taking place in the modified functional assay were stopped at intervals with EA anticoagulant and subjected to the FVIIa ELISA (Fig 4). It is evident that with prolonged incubations activation of FVII does occur.
FVII activation in the FVIIa functional activity assay. A modification of the functional activity assay was performed using plasma that had been depleted of fibrinogen. Overall reagent concentrations were as described for the functional assay. Plasma was incubated with the functional assay reagents until the reactions were terminated by the addition of an anticoagulant cocktail (see text). The products of the reactions at varying time points were then assayed for FVIIa using the ELISA.
FVII activation in the FVIIa functional activity assay. A modification of the functional activity assay was performed using plasma that had been depleted of fibrinogen. Overall reagent concentrations were as described for the functional assay. Plasma was incubated with the functional assay reagents until the reactions were terminated by the addition of an anticoagulant cocktail (see text). The products of the reactions at varying time points were then assayed for FVIIa using the ELISA.
Effect of Freezing and Thawing of Plasma on FVIIa Levels
Plasma samples taken from control individuals (n = 14) were assayed for FVIIa levels before and after one round of a freeze/thaw cycle. FVIIa measurements in normal fresh plasma were 0.038 ± 0.015 ng/mL (mean ±SE), following a freeze/thaw cycle they were 0.039 ± 0.014 ng/mL (P = .95).
Investigation of Artifactual Generation of FVIIa During Venepuncture
Two consecutive vacutainers of blood were taken during a single venepuncture from normal individuals (n = 14). Plasma from both vacutainers were assayed fresh and after a freeze/thaw cycle. There was no significant difference in ELISA results between the first and second withdrawal of blood, 0.038 ± 0.014 ng/mL cf 0.025 ± 0.011 ng/mL (mean ±SE), n = 14, NS.
Relationship Between FVIIa Levels and Prothrombin Time (PT)
Fresh plasma samples from normal laboratory workers (n = 14) were assayed for immunologic-determined FVIIa level and the PT. We found no correlation between PT and FVIIa determined with the immunoassay or between the PT and results obtained with results of the functional FVIIa assay in this small sample cohort (these results are not illustrated).
FVIIa Levels in the Acute Coronary Syndromes
Results of FVIIa and F1 + 2 levels in age-matched patients presenting with noncardiac chest pain, acute MI, and unstable angina can be seen in Fig 5A and B and Table 4. Elevations in FVIIa and F1 + 2 occur in acute coronary syndromes (MI and unstable angina grouped together) when compared to the noncardiac chest pain control group (for FVIIa compare 0.093 ± 0.013 ng/mL with 0.048 ± 0.007 ng/mL, P < .05; for F1 + 2 compare 29.6 ± 3.1 ng/mL with 18.7 ± 1.9 ng/mL, P < .05 — results expressed here as mean ± SE). Statistical significance of elevations above the noncardiac chest pain group (using the Mann Whitney-Wilcoxon test) is also achieved individually in the unstable angina patients for FVIIa and in the acute MI patients for F1 + 2. Correlation analysis between FVIIa and F1 + 2 results in the acute coronary syndromes yields a Spearman correlation coefficient (rs ) of 0.4 (P < .01), demonstrating a relationship exists between immunologic-determined FVIIa and thrombin generation in the acute coronary syndromes.
(A) Individual distribution of FVIIa results determined by ELISA in patients presenting with noncardiac chest pain (NCP), unstable angina (UAP), and myocardial infarction (MI). The significance of differences between results of noncardiac chest pain group and (A) unstable angina group, and (B) all patients with acute coronary syndromes, is indicated by the P value. (B) Individual distribution of F1 + 2 results determined by ELISA in patients presenting with noncardiac chest pain (NCP), unstable angina (UAP), and myocardial infarction (MI). The significance of differences between results of noncardiac chest pain group and (A) myocardial infarction group, and (B) all patients with acute coronary syndromes, is indicated by the P value.
(A) Individual distribution of FVIIa results determined by ELISA in patients presenting with noncardiac chest pain (NCP), unstable angina (UAP), and myocardial infarction (MI). The significance of differences between results of noncardiac chest pain group and (A) unstable angina group, and (B) all patients with acute coronary syndromes, is indicated by the P value. (B) Individual distribution of F1 + 2 results determined by ELISA in patients presenting with noncardiac chest pain (NCP), unstable angina (UAP), and myocardial infarction (MI). The significance of differences between results of noncardiac chest pain group and (A) myocardial infarction group, and (B) all patients with acute coronary syndromes, is indicated by the P value.
DISCUSSION
The importance of extrinsic pathway activation of coagulation has become increasingly recognized. However, the role that FVIIa plays in hemostasis and thrombosis has not been clearly defined in part because of a lack of adequate methodology for its investigation. To date the most specific means of estimating FVIIa levels in plasma has been with a functional activity assay using truncated tissue factor capable of serving as a cofactor for FVIIa but not for FVII. An immunoassay has been unavailable because of the difficulty in obtaining an antibody that specifically recognizes FVIIa without cross-reacting with FVII. Using a synthetic peptide immunogen based on the amino acid sequence N-terminal to the activation cleavage site of FVII that exposes the C-terminus, we successfully raised a unique antibody clearly able to discriminate (>3,000-fold) between FVIIa and FVII at physiologic concentrations, thereby facilitating the development of a novel immunoassay to FVIIa. A similar approach has been described in abstract form only by others.16
This immunoassay designed in the form of an ELISA was developed using two tag antibodies, monoclonal or polyclonal antibodies raised to FVII (which also recognize FVIIa). Results obtained using either tag antibody were virtually identical. Biotinylation of the tag antibody enabled signal amplification in the assay, which was further increased with a commercial enzyme amplification kit. Evaluation of the ELISA showed excellent sensitivity, reproducibility, linearity, with satisfactory recovery of human FVIIa across a wide range of concentrations.
A major discrepancy exists between the ELISA and functional FVIIa assay when endogenous plasma concentrations of FVIIa are measured in normal subjects. Levels of FVIIa in normal laboratory controls (n = 14) were 3.10 ± 0.30 ng/mL (mean ± SE) for the functional assay and 0.025 ± 0.010 ng/mL for the immunoassay. Our results for the functional FVIIa assay are in broad agreement to those published by others. Morrissey et al12 estimated the mean plasma level of FVIIa to be 0.76% of total FVII (assuming a mean concentration of plasma FVII to be 470 ng/mL20 ). Our ELISA results predict that only 0.006% of total FVII circulates in its activated form. Possible explanations for this ∼100-fold difference in estimated endogenous plasma levels are that functionally active FVIIa may be bound to lipid or to tissue factor and therefore inaccessible for antibody recognition. However, FVIIa levels did not change in the presence of detergent or of soluble tissue factor. The remaining plausible explanation for the discrepancy between the two methods is recruitment of additional FVIIa from FVII zymogen occurring during the functional assay. It is already established that truncated tissue factor does not inhibit the tissue factor–independent activation of FVII by FXa.10 If small amounts of FVII become activated during the functional assay, either by proteinases or by truncated tissue factor-FVIIa complex, artifactual high levels of FVIIa will be obtained. Investigation of the products of a modified functional assay using our ELISA shows that prolonged incubation of plasma with truncated tissue factor does give rise to FVII activation. Complete resolution of this point has been hindered by the extremely low levels detected by the ELISA, making experimental manipulation and exploration difficult. A further point to be considered is the calibration of the functional assay. The standard curve is constructed with a range of recombinant FVIIa concentrations added to FVII-depleted plasma (ie, in the absence of FVII), whereas plasma samples (for investigation) contain FVII and are diluted 1 in 10 in FVII-depleted plasma. As the values for FVIIa sample levels are multiplied 10-fold to correct for the dilution with FVII-deficient plasma, any activation of FVII arising during the course of the functional assay will therefore be enhanced 10-fold by this computation. These results and considerations strongly suggest that the functional assay results may be influenced by in vitro activation of FVII. If correct, this explanation would explain the differences in level of endogenous plasma FVIIa detected with the two assays, while allowing the excellent relationship observed when increasing amounts of FVIIa were added to plasma.
Epidemiological studies have suggested that plasma FVII:C and fibrinogen levels are risk factors for ischemic heart disease and cardiovascular death.5,21,22 The FVIIa ELISA may be used to examine the role of FVIIa in prospective studies of cardiovascular diseases. A preliminary investigation of the relationship between FVIIa and F1 + 2 levels in acute cardiovascular disease is reported in the current study. In patients presenting with acute MI and unstable angina, activation of coagulation is evident as seen by FVIIa and F1 + 2 levels when compared with an age-matched group of noncardiac chest pain patients. Although these patients with acute coronary syndromes collectively have elevated levels of these coagulation markers, it is of interest to note that many have levels within the normal range. In respect to F1 + 2 levels, these findings of broad overlap of levels between patients and controls, but overall elevation in patients, agree with those results published by Merlini et al,23 who were the first to demonstrate increased F1 + 2 in this situation.24 [In a recent evaluation of the prognostic significance of admission F1 + 2 levels in acute coronary syndromes, we have shown that patients presenting with F1 + 2 levels outside the range found in laboratory controls have significantly increased mortality determined over a 3-year follow-up (unpublished observations, June 1996).] However, Merlini et al23 reported no increase in FVIIa levels (determined with a functional activity assay based on truncated tissue factor) in the acute coronary syndromes, when comparison was made to results of patients with stable angina and healthy controls. The methodologic limitations of the functional assay noted above may have masked a small increase in FVIIa levels. In the preliminary study reported here, we provide evidence that FVIIa may indeed be elevated, when it is detected by the FVIIa ELISA. Furthermore, using correlation analysis, we have been able to show a statistically significant relationship between FVIIa and F1 + 2 levels in the acute coronary syndromes. As FVIIa and F1 + 2 differ in nature (compare proteinase with activation fragment) and in position in the coagulation cascade, a near-perfect relationship between the two would be unexpected. The results, taken together with our current understanding of the biochemistry of blood coagulation, do suggest therefore a possible causative relationship between activation of FVII and generation of thrombin in acute coronary syndromes. This finding would support the current belief that extrinsic pathway activation is responsible for triggering coagulation in acute cardiovascular events, although further studies are required to exclude the possibility that activation of FVII may be a secondary event following thrombin generation.
In summary, we have developed a novel immunoassay for FVIIa which has the potential to be used to identify FVII activation in situations of provoked coagulation activation. Use of this assay may enable trigger mechanisms of coagulation in thrombosis to be elaborated.
Supported by a grant from the British Heart Foundation to D.A.L.
Address reprint requests to David A. Lane, PhD, Department of Haematology, Charing Cross and Westminster Medical School, Hammersmith, St Dunstan's Rd, London W6 8RP, UK.