Abstract
Transplantation of hematopoietic stem cells from an HLA-compatible unrelated volunteer is an option for patients with acute leukemia lacking a family match. However, criteria for patient and donor selection and the most effective transplant procedures, including the number of hematopoietic cells, remain to be defined. We tested factors influencing outcome of 174 patients with primary acute leukemia receiving non–T-cell depleted marrow from unrelated donors. Median patient age was 20 years (range, 0.5 to 54 years). A multivariable analysis found that leukemia in remission at the time of transplantation was associated with improved leukemia-free survival (relative risk [RR] of treatment failure: 0.5, confidence interval [CI]: 0.3 to 0.7), and presence of blasts in the peripheral blood, as opposed to marrow involvement only or isolated extramedullary relapse, was associated with impaired outcome (RR of treatment failure: 2.5, CI: 1.7 to 5.0). The use of donors with a limited HLA-mismatch was associated with decreased leukemic relapse (RR: 0.5, CI: 0.3 to 0.9) but no improvement in leukemia-free survival compared with HLA-matched unrelated donors. Transplantation of a marrow cell dose above the median value of 3.65 × 108/kg was associated with faster neutrophil (RR: 1.5, CI: 1.1 to 2.0) and platelet (RR: 4.5, CI: 2.7 to 7.5) engraftment, and decreased incidence of severe acute graft-versus-host disease (RR: 0.6, CI: 0.4 to 0.9). In patients transplanted in remission, the use of a marrow cell dose above the median translated into less nonleukemic death (RR: 0.2, CI: 0.1 to 0.4) and better leukemia-free survival (RR of treatment failure: 0.3, CI: 0.2 to 0.6). Transplant in remission with a high dose of marrow cells was associated with the best outcome in both children and adults.
MARROW TRANSPLANTATION from an HLA histocompatible donor is the only potentially curative therapy for many patients with acute leukemia.1 The antileukemic activity of allogeneic marrow transplantation is provided both by the high-dose intensity of the conditioning regimen and by an immune mediated graft-versus-leukemia reaction.2 Although only 30% of patients have a HLA compatible sibling, the development of a registry network including more than 3 million HLA-typed volunteers, has extended the availability of allogeneic transplantation to patients without a family match. In the United States, the probability of finding at least an HLA-A, B, DR matched donor within the National Marrow Donor Program (NMDP) has increased from less than 10% in 1987 to 64% in 1995.3 Marrow transplantation from an HLA compatible unrelated donor has been performed for many patients with high-risk or advanced stage acute leukemia,4-11 but this procedure has been associated with high morbidity and mortality caused by poor engraftment, graft-versus-host disease (GVHD) and associated infectious complications.
We have analyzed factors that have affected outcome of patients with acute leukemia treated with unrelated donor marrow transplants in Seattle during the last 15 years. We found improved survival with transplantation early in the course of the disease and with low tumor burden. We confirmed that donors with limited HLA mismatch can be used without jeopardizing survival, at least in younger patients. A higher dose of marrow cells was associated with improved hematological recovery and lower incidence of severe acute GVHD. In patients transplanted in remission, the use of a high marrow cell dose translated into lower transplant-related mortality and improved leukemia-free survival.
MATERIALS AND METHODS
Patients.Between September 4, 1979 and June 30, 1994, 204 consecutive patients with acute leukemia received unrelated donor marrow transplants at the Fred Hutchinson Cancer Research Center (n = 199) or the Seattle Veterans Affairs Medical Center (n = 5). This report excludes patients with leukemia secondary to myelodysplasia evolving over a period longer than 4 months (n = 12), leukemia secondary to chemotherapy or radiation (n = 7), recurrent leukemia after an autologous transplant (n = 9), and patients with acute leukemia transplanted with T-cell depleted marrow (n = 2). The remaining 174 patients with primary acute leukemia form the study cohort (Table 1). Forty-five (25%) patients received an unrelated transplant before 1990.
Disease characteristics.Table 1 shows patient and donor characteristics according to status of leukemia at transplant. Results of cytogenetic studies of marrow cells at diagnosis were available in 44 (59%) of 74 acute myeloid leukemia (AML) patients, 44 (48%) of 91 acute lymphoid leukemia (ALL) patients, and 5 (55%) of 9 patients with hybrid leukemia. Chromosomal abnormalities classified as good prognosis features included AML with inversion 16 (n = 1), translocation 8; 21 (n = 2) or 15; 17 (n = 2), and ALL with hyperdiploid karyotype (n = 6). The karyotypes classified as having poor prognosis included AML with abnormalities of chromosomes 5 (n = 2) or 7 (n = 4), those involving the long arm of chromosome 11 (n = 2) or translocation 6; 9 (n = 1), and ALL or hybrid leukemia with translocation 1; 19 (n = 4), t 4; 11 (n = 8) or t 9; 22 (n = 10). The other 53 patients (including 25 with a normal karyotype) were assigned to an intermediate risk category.
All 11 patients transplanted in first complete remission had poor prognosis features: unfavorable cytogenetics (n = 6) consisting of t 9; 22 (n = 4), t 4; 11 (n = 1), and −7 abnormality (n = 1), more than two courses of chemotherapy required to achieve complete remission (n = 3), congenital leukemia (n = 1), or persistent dysplasia (n = 1). Thirty-five (83%) of the 42 patients transplanted in first relapse, and 16 (46%) of the 35 patients transplanted in second remission had a duration of the first remission less than 1 year. In 18 (43%) of 42 patients in first relapse, no attempt was made to obtain another remission, whereas 24 (57%) individuals were transplanted in chemotherapy-resistant relapse. Thirty-two (61%) of 52 patients were transplanted in untreated second or subsequent relapse and 20 (39%) after treatment for relapse. Before starting the conditioning regimen, four (5%) of 94 patients transplanted in relapse had isolated extramedullary leukemia without blasts in marrow or blood, 19 (20%) patients had less than 30% blasts in marrow with no detectable blasts in blood, 18 (19%) had ≥30% or more blasts in marrow and not in blood, and 53 (56%) patients had blasts in the peripheral blood.
Histocompatibility studies and donor selection.Unrelated donor marrow was provided by Donor Centers associated with the NMDP, Minneapolis, MN (n = 138), Europdonor Foundation, Leiden, The Netherlands (n = 8), Anthony Nolan Registry, London, UK (n = 17), France Greffe de Moelle Registry, Paris, France (n = 7), German National Registry, Ulm, Germany (n = 3), and Australian Bone Marrow Donor Registry, Sidney, Australia (n = 1). The median interval time between initiation of the unrelated donor search and transplant was 5.3 months (range, 1 to 68 months). HLA-typing for all patients and donors was confirmed by the Fred Hutchinson Cancer Research Center Clinical Immunogenetics Laboratory. Histocompatibility studies for class I antigens consisted of serological typing according to a standard two-stage microtoxicity assay,12 and antigens were assigned as defined by the World Health Organization HLA nomenclature committee effective at the time of transplantation.13 In 151 (87%) pairs, class II region compatibility was assessed by DRB1 allele typing by hybridization of amplified DNA with sequence-specific oligonucleotide probes (SSOP).14 In the remaining 23 pairs (13%), HLA-DR typing was performed with nylon-wool purified B cells in a modified microtoxicity crossmatch assay12 and “Dw” assignments were made by stimulation with HLA-D homozygous typing cells in a standard HLA-D typing assay.13 Donor and recipient pairs were considered matched when identical at HLA-A, B, and D/DRB1 loci. Minor mismatched pairs were those with a single disparity for class I antigens belonging to the same crossreactive group as defined by the NMDP, or for a single disparity for D/DRB1 subtypes alleles within the same DR specificity (DR1, 15[2], 16[2], 3, 4, 11[5], 12[5], 13[6], 14[6], 7, 8, 9, 10). Patients unable to find an HLA-identical donor were eligible for a one antigen minor mismatch if they were less than 36 years old. The distribution according to HLA-matching appears in Table 1.
Transplantation procedure.The Institutional Review Board of the Fred Hutchinson Cancer Research Center approved all treatment protocols. Written informed consent was obtained from adult patients and from parents or guardians of children less than 18 years old. Conditioning for transplantation consisted of cyclophosphamide, 60 mg per kg recipient body weight, intravenously on each of 2 successive days and total body irradiation (TBI) in 168 (96%) cases. Hyperfractionated TBI was administered from dual opposing 60 Co sources to a total dose of 12 Gy in 4 (2%) cases, 13.2 Gy in 94 (53%), 14.4 Gy in 64 (37%), and 15.75 Gy in 6 (4%). Preparative treatment with chemotherapy alone was administered to 6 (4%) patients.
Marrow cells were transported from the harvest center at room temperature or at 4°C if the transit time was expected to exceed 12 hours and were administered intravenously after completion of the conditioning regimen. When major ABO incompatibility was present, plasmapheresis or marrow red blood cell depletion was performed before marrow infusion. In case of minor ABO incompatibility, plasma was removed from the marrow before the infusion. Through June 30, 1991, the dose of marrow prescribed to patients at Seattle was 15 mL per kg recipient body weight. The median marrow cell dose infused to 84 patients treated until that date, on this study was 3.82 × 108 cells per kg. Starting July 1, 1991, the NMDP changed its policy and began to require a specified marrow cell dose in each marrow prescription. We started to request 4 × 108 cells per kg recipient body weight. For the second cohort of 90 patients on this study, the median marrow cell dose was 3.6 × 108 per kg. For the whole study, the number of nucleated marrow cells (not corrected for potential contamination with peripheral blood cells) ranged from 0.7 to 46.1 (median, 3.65) × 108/kg recipient body weight. The median number of nucleated cells infused to patients less than 18 years of age was 4.8 (range, 1.4 to 46.1) × 108/kg and to those 18 or greater was 3.3 (range, 0.7 to 9.9) × 108/kg. Twenty-nine percent of patients less than 18 and 64% of those ≥18 received a marrow cell dose below 3.65 × 108/kg, that is the median value for the whole series. One hundred thirty-four patients (77%) were transplanted in laminar air flow rooms and in the remaining 40 in conventional rooms. Prophylactic intravenous antibiotics were administered since the beginning until the end of the period of neutropenia below 0.5 × 109/L. Thirteen patients (8%) received granulocyte-macrophage colony stimulating factors (GM-CSF ) after transplant as part of research protocols. Acute GVHD prophylaxis in 148 (85%) cases consisted of standard cyclosporine and methotrexate according to the schedule reported elsewhere.15
Engraftment.Myeloid engraftment was defined as the first of 3 consecutive days when the absolute neutrophil count (ANC) surpassed 0.5 × 109/L. Primary graft failure was defined as the lack of myeloid engraftment in patients surviving in remission for at least 28 days after transplantation. Secondary graft failure was defined as recovery followed by a sustained decrease of ANC to below 0.10 × 109/L. Platelet engraftment was defined as the first of 7 consecutive days when the platelet count exceeded 50 × 109/L without transfusion support.
Acute and chronic GVHD.Acute and chronic GVHD were diagnosed and graded according to consensus criteria.16 All patients were considered evaluable for acute GVHD by a time-dependent analysis. Primary treatment for acute GVHD consisted of glucocorticoids or monoclonal or polyclonal anti–T-cell antibodies.17-19 The occurrence of chronic GVHD was evaluated among patients who survived in remission for at least 100 days after transplantation. Patients without evidence of extensive chronic GVHD were began on tapering their immunosuppressive therapy with discontinuation of all immunosuppressive agents by day 180 if they remained free of signs or symptoms of chronic GVHD. Patients with clinical extensive chronic GVHD were continued on immunosuppressive therapy for at least an additional 9 months.20 21
Statistical analysis.Results of the study were analyzed as of June 30, 1995. Raw proportions were compared by using the chi-square test. Prognostic factors influencing leukemia-free survival, nonleukemic death, and relapse were analyzed in the entire group of 174 cases and separately in 66 patients transplanted during remission and in 94 patients transplanted during relapse. The 27 variables listed in Table 2 were evaluated in a univariable prognostic factor analysis comparing Kaplan-Meier curves.22 Those parameters reaching a significance level ≤0.1 in the log-rank test were included in a multivariable analysis using the Cox regression method.23 Numeric variables were analyzed as continuous distributions or as categories considering their value above or below the median of the series as indicated in the Results. All analyses were corrected for the type of leukemia (ALL v AML v hybrid leukemia). Multivariable analyses were also performed to investigate the parameters associated with granulocyte and platelet engraftment, development of grades III-IV acute GVHD and extensive chronic GVHD in the entire group of 174 patients. In addition to marrow cell dose, the variables analyzed were age, gender and pretransplant cytomegalovirus (CMV) serology of donor and recipient, obesity, type of leukemia, status of the disease at the time of transplant, and HLA-matching. Acute GVHD was also included as covariate when analyzing the development of extensive chronic GVHD. The cumulative incidence of granulocyte recovery, platelet recovery, acute GVHD, chronic GVHD, nonleukemic death, relapse, and leukemia-free survival were calculated.24 The onset of acute GVHD was defined by the first clinical sign of GVHD during the first 100 days after transplant. The onset of chronic GVHD was defined by the day when the diagnosis of clinical extensive chronic GVHD was made. The definitions of leukemia-free survival, nonleukemic death, and relapse have been reported elsewhere.25P values appearing in the figures represent the results of log-rank test.
RESULTS
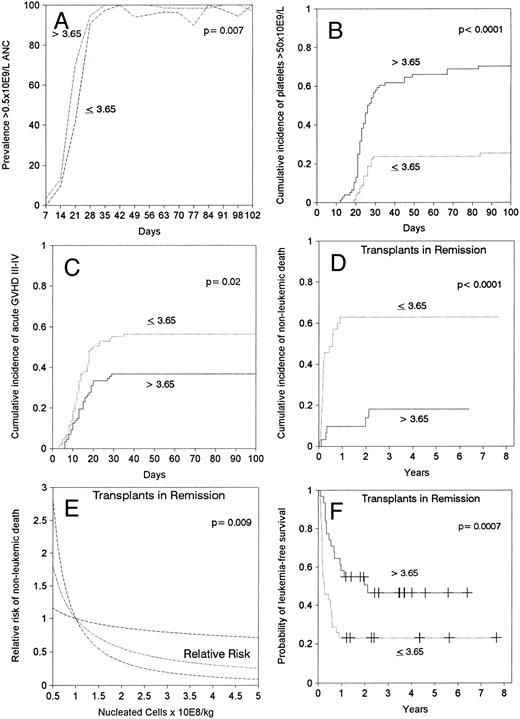
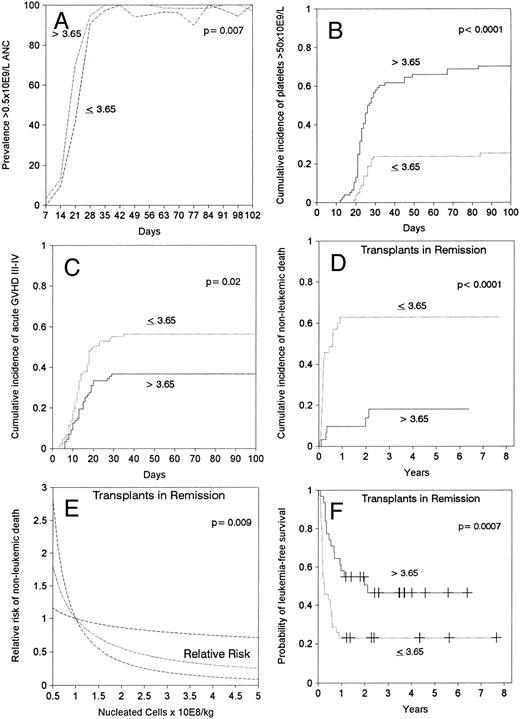
Engraftment.Sixteen patients (9%) died during the first 28 days after transplantation, six of them with a neutrophil count above 0.5 × 109/L. All but two (1%) of the remaining 158 patients achieved sustained donor engraftment. One patient had primary failure of engraftment and one had secondary graft failure. ANC surpassed 0.5 × 109/L at a median of 21 days after transplantation. In multivariable analysis, faster neutrophil recovery was associated with a marrow cell dose above the median of 3.65 × 108 nucleated cells/kg (relative risk [RR]: 1.5, confidence interval [CI]: 1.1 to 2.0, P = .01, Fig 1A), and transplant in remission (RR: 1.5, CI: 1.1 to 2.1, P = .01). Episodes of neutropenia below 0.5 × 109/L between days 42 and 84 after transplantation occurred in 13 (15%) patients in the low cell dose group and in two (2%) of those in the high cell dose group (Fig 1A). A marrow cell dose above 3.65 × 108 nucleated cells/kg also lead to faster recovery of 0.5 × 109/L lymphocytes (median, 25 days v 50 days, P = .002). Self-sustained platelet counts surpassed 50 × 109/L by 100 days in 48% of patients, median time would be greater than 100 days. Platelet recovery occurred earlier in patients receiving >3.65 × 108 marrow nucleated cells/kg (RR: 4.5, CI: 2.7 to 7.5, P < .001, Fig 1B) and in those transplanted in remission (RR: 2.6, CI: 1.7 to 4.2, P < .001).
Effect of marrow cell dose (nucleated cells × 108/kg of recipient body weight) on (A) prevalence of neutrophil count above 0.5 × 109/L, (B) cumulative incidence of achieving a self-sustained platelet count greater than 50 × 109/L, (C) cumulative incidence of grade III-IV acute GVHD, (D) cumulative incidence of nonleukemic death after transplant in remission, (E) estimated RR of nonleukemic death in transplants in remission showing a linear decrease in the RR by increasing marrow cell dose (the dashed lines limit 95% CI), (F ) leukemia-free survival after transplant in remission.
Effect of marrow cell dose (nucleated cells × 108/kg of recipient body weight) on (A) prevalence of neutrophil count above 0.5 × 109/L, (B) cumulative incidence of achieving a self-sustained platelet count greater than 50 × 109/L, (C) cumulative incidence of grade III-IV acute GVHD, (D) cumulative incidence of nonleukemic death after transplant in remission, (E) estimated RR of nonleukemic death in transplants in remission showing a linear decrease in the RR by increasing marrow cell dose (the dashed lines limit 95% CI), (F ) leukemia-free survival after transplant in remission.
Acute and chronic GVHD.The cumulative incidence rates of grades II-IV or III-IV acute GVHD were 82% and 47%, respectively, and the median time of onset was 15 days. A marrow cell dose above the median value of 3.65 × 108/kg did not affect the incidence of grade II-IV acute GVHD, but was the only factor associated with lower incidence of grade III-IV acute GVHD (RR: 0.6, CI: 0.4 to 0.9, P = .01, Fig 1C). A cell dose above 3.65 × 108/kg was associated with a lower incidence of grade III-IV GVHD both in patients <18 years of age (37% v 68%), and in patients 18 or older (36% v 51%). Ninety-two (53%) patients survived in remission for at least 100 days after transplantation and were at risk for chronic GVHD. Fifty-two percent of evaluable patients developed clinical extensive chronic GVHD with onset at a median of 206 days after transplantation. By multivariable analysis, older patient age (RR: 1.03, CI: 1.01 to 1.05, P = .01) and previous development of grades III-IV acute GVHD (RR: 1.8, CI: 1.04 to 3.3, P = .04) were associated with an increased incidence of clinical extensive chronic GVHD. The marrow cell dose did not affect the incidence of chronic extensive GVHD.
Nonleukemic death.The 5-year cumulative mortality from causes other than leukemic relapse was 39%, with 73% of these events occurring within the first 100 days and 27% thereafter. Twenty-eight (57%) of the 49 patients who died during the first 100 days had been diagnosed with grades III-IV acute GVHD, and 14 (78%) of the 18 patients who died after day 100 had developed clinical extensive chronic GVHD (P = .10). Marrow cell dose above the median was the only significant factor associated with a lower risk of nonleukemic death in the entire group of 174 patients, as a reflection of what was observed in the 66 patients transplanted in remission (Fig 1D and Table 3). For the latter, the association of marrow cell dose and nonleukemic death remained significant when marrow cell dose was introduced in the models as a continuous variable (Fig 1E). Patient age was not a significant factor in any of the multivariable analyses that considered the effect of marrow cell dose. Among the group transplanted during remission, higher marrow cell doses were associated with a lower cumulative incidence of nonleukemic death both in the 33 patients <18 years of age (64% v 15%, P < .001) and in the 33 patients ≥18 years of age (63% v 24%, P = .01). The effect of marrow cell dose could not be explained by a worse prognosis for obese patients (body weight/ideal body weight >1.25) because this parameter had no predictive value in multivariable analyses and because the effect of cell dose remained significant after obese patients were excluded (data not shown). Patients in remission transplanted with low cell doses died more frequently of bacterial or fungal infection (32% v 3%, P = .006) than those transplanted with a higher cell dose (Table 4).
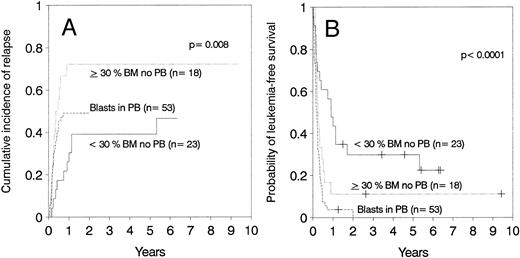
Marrow cell dose showed no significant association with nonleukemic death among patients transplanted during relapse (Table 4). In this group, the presence of blasts in the peripheral blood before starting the conditioning regimen was the single factor associated with an increased risk of nonleukemic death (Table 3). Twenty-six (49%) of 53 patients with circulating blasts died due to causes other than leukemia versus 10 (24%) of 41 patients in relapse without circulating blasts at the time of transplantation (Table 5).
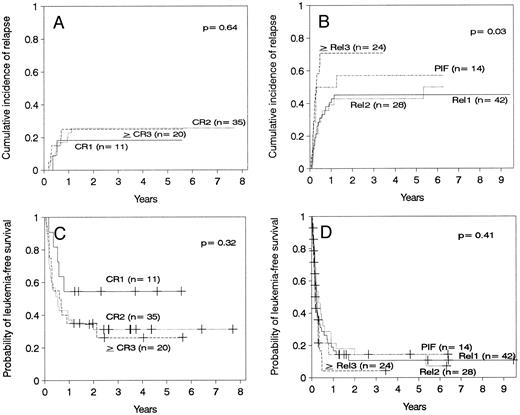
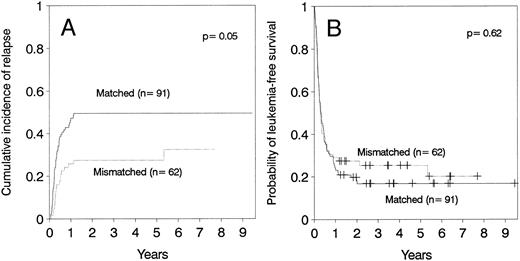
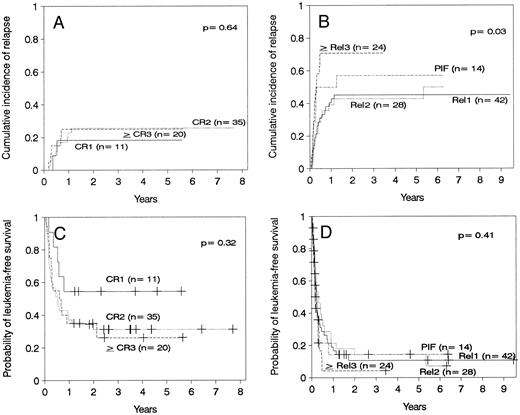
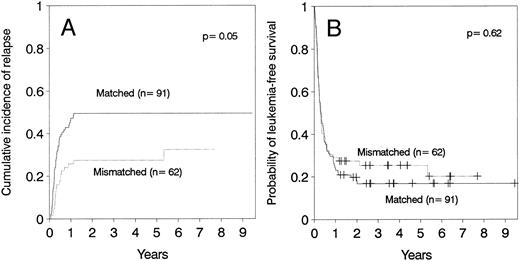
Relapse.The cumulative incidence of relapse after transplantation was 41% with a median onset at 9 months (range, 0.7 months to 5.5 years). Marrow cell dose was not associated with the risk of relapse after transplantation. Patients transplanted during remission had a significantly lower risk of leukemic relapse than those transplanted in relapse or after primary induction failure (Table 3, Figs 2A and B). Patients with ≥30% leukemic blasts in the marrow before starting the conditioning regimen had higher risk of relapse after transplant than patients in relapse with <30% blasts in the marrow (Table 3 and Fig 3A). Patients transplanted during third or subsequent relapse had a 71% cumulative incidence of posttransplant relapse versus 43% for those transplanted during second relapse and 45% for those transplanted during first relapse (Fig 2B and Table 3). Patients transplanted from HLA-mismatched donors had a lower incidence of recurrent leukemia than those transplanted from matched donors (Table 3, Fig 4A). Among the 66 patients transplanted during remission, the use of an HLA-mismatched donor was the single factor associated with a decreased risk of recurrent malignancy after transplantation (RR: 0.2, CI: 0.05 to 1.1, P = .06).
(A) Cumulative incidence of relapse of patients transplanted in remission. (B) Cumulative incidence of relapse of patients transplanted with active leukemia. (C) Leukemia-free survival of patients transplanted in remission. CR1, first remission; CR2, second remission; ≥CR3, third or subsequent remission. (D) Leukemia-free survival of patients transplanted with active leukemia. Rel1, first relapse; Rel2, second relapse, ≥Rel3, third or subsequent relapse; PIF, primary induction failure.
(A) Cumulative incidence of relapse of patients transplanted in remission. (B) Cumulative incidence of relapse of patients transplanted with active leukemia. (C) Leukemia-free survival of patients transplanted in remission. CR1, first remission; CR2, second remission; ≥CR3, third or subsequent remission. (D) Leukemia-free survival of patients transplanted with active leukemia. Rel1, first relapse; Rel2, second relapse, ≥Rel3, third or subsequent relapse; PIF, primary induction failure.
(A) Cumulative incidence of relapse and (B) leukemia-free survival depending on the number of blasts in marrow (BM) or blood (PB).
(A) Cumulative incidence of relapse and (B) leukemia-free survival depending on the number of blasts in marrow (BM) or blood (PB).
(A) Cumulative incidence of relapse and (B) leukemia-free survival according donor HLA-matching in patients ≤36 years of age.
(A) Cumulative incidence of relapse and (B) leukemia-free survival according donor HLA-matching in patients ≤36 years of age.
Leukemia-free survival.Thirty-four (19%) of the 174 patients remain alive and disease-free with a median follow-up of 3.5 years (range, 1.2 to 9.6 years). Twenty-five of the 34 patients (73%) have a Karnofsky score of 100%, 4 (12%) between 90% and 99%, and 5 (15%) below 90%. Seven patients continue on treatment for chronic GVHD for periods ranging between 16 and 69 months, whereas 27 (79%) have discontinued immunosuppressive treatment.
Leukemia-free survival was not significantly different for patients with ALL versus AML or hybrid leukemia (P = .21). In particular, leukemia-free survival at 5 years was 27% ± 11% for 15 patients with AML in second remission and 37% ± 11% for 19 patients with ALL in second remission (P = .7). Leukemia-free survival at 5 years was 12% ± 5% in 40 cases of AML transplanted in relapse and 10% ± 4% in 49 cases of ALL also transplanted in relapse (P = .7). Other patients groups are too small for comparison. The presence of cytogenetic abnormalities was not associated with outcome in the 93 patients with data available. Patients transplanted during remission had better leukemia-free survival than those transplanted in relapse or after primary induction failure (Fig 2C and D, Table 3). Among patients transplanted during relapse, those with less than 30% blasts in the marrow and no blasts in the blood had a 5-year leukemia-free survival of 30% ± 10%, those with ≥30% blasts in the marrow and no blasts in the blood had a 5-year leukemia-free survival of 11% ± 7%, and patients with blasts in the blood had a leukemia-free survival of 0% at 2 years (Fig 3B). We could not find an advantage for transplantation of patients in early “untreated” relapse rather than chemotherapy-refractory relapse. Leukemia-free survival at 5 years was 12% ± 5% for 50 patients transplanted in untreated relapse versus 5% ± 4% for 44 patients transplanted in chemotherapy-refractory relapse (P = .22). When the analysis was restricted to patients in first relapse, leukemia-free survival at 5 years was 11% ± 7% for 18 patients transplanted in untreated relapse versus 11% ± 6% for 24 patients in chemotherapy-refractory relapse (P = .66). Higher cell dose was significantly associated with better leukemia-free survival in the multivariable analysis (Table 3) and remained the single factor significantly associated with improved survival among patients transplanted during remission (Fig 1F ). This finding was independent of patient obesity and age. In particular, a cell dose above 3.65 × 108/kg was associated with higher leukemia-free survival both in patients <18 years of age (46% v 30%), and in patients 18 or older (52% v 17%). Consistent with previous reports, leukemia-free survival was better for patients with negative CMV serology before transplant (Table 3).
DISCUSSION
This study confirms and extends previous findings that transplantation of hematopoietic stem cells from an HLA-compatible unrelated donor can result in long-term leukemia-free survival in patients with otherwise incurable acute leukemia such as those with primary induction failure.4,5,6,8,11 Six of 11 leukemia patients in first remission and poor prognosis features are alive and leukemia-free between 1.2 and 5.6 years (median, 3 years) after transplant. Despite the small number of patients, these results encourage us to consider an unrelated donor marrow transplant as curative consolidation therapy for acute leukemia in first remission in patients at high-risk of relapse with conventional treatment. For patients beyond first remission, better survival was associated with transplantation during a second or third complete remission. Patients in relapse with less than 30% marrow blasts in the marrow had a relatively low risk of relapse after transplantation and a probability of leukemia-free survival similar to that of patients transplanted in second or third remission. Conversely, patients with greater than 30% blasts in the marrow or with any blasts in the blood had transplant-related mortality and relapse that approached 100%. New treatment strategies are required to decrease relapse with lower toxicity. Promising new approaches include the use of leukemia specific immunotoxins or radiolabeled monoclonal antibodies in the pretransplant conditioning regimen.26
This study demonstrates that HLA-disparity is associated with a lower risk of leukemia relapse, particularly in patients transplanted during remission. However, HLA-disparity is also associated with a higher risk of acute GVHD and transplant-related mortality27 thereby obviating any overall survival advantage. Nevertheless, the ability to use HLA-mismatched donors for patients up to age of 36 years, as defined in this study, increases the proportion of patients who can be transplanted.
A new finding of our study was that transplantation of a large marrow cell dose was associated with faster and more durable hematological recovery and reduced severity of acute GVHD. Higher cell dose was associated not only with improved engraftment of granulocyte and platelets, but also with faster lymphocyte recovery. A significant reduction in mortality due to infections was observed in patients in remission receiving a higher number of marrow cells. Although children received, on average, a higher marrow cell dose than adults, the decrease in transplant-related mortality associated with high marrow cell dose was significant when children and adults were analyzed separately. In addition, age was not a significant covariate with marrow cell dose in the multivariable analyses. Therefore, the favorable effects attributed to marrow cell dose in this study were not a simple reflection of an improved outcome with younger age. These findings, therefore, suggest that a lower marrow cell dose may account at least in part for the poorer outcome of adults compared with children.
Transplantation of a high marrow cell dose might not prevent death from infections present in neutropenic patients before the start of the conditioning regimen. This concept could explain why the effect of marrow cell dose on nonleukemic death was observed in patients transplanted in remission, but not in those in relapse. In fact, among the six patients transplanted in relapse who died of infection despite receiving a high marrow cell dose, five had an ANC below 0.5 × 109/L before transplant, and four also had fever or documented fungal disease. Reports of related donor marrow recipients have shown that a higher cell dose was associated with a lower incidence of graft rejection and a higher speed of engraftment.28-32 Two studies have also found that a higher cell dose improved survival after related donor transplant for acute leukemia.33,34 In one International Bone Marrow Transplant Registry study, patients receiving higher cell dose had less severe GVHD.33 A possible explanation for this finding is that a higher cell dose leads to a decreased incidence of early posttransplant infections, which may amplify GVHD. This argument has also been used to explain why a higher dose of spleen cells is associated with lower GVHD and mortality in major histocompatibility complex-incompatible murine transplants.35
Data from our study lead to the hypothesis that improved leukemia-free survival could be achieved by increasing the number of stem cells in the graft. A randomized study of GM-CSF administered early after transplant failed to show a survival benefit, but it remains to be tested whether the use of other hematopoietic growth factors may provide an advantage.36 Because the number of harvestable marrow cells is limited, one could consider blood as an additional source of hematopoietic stem cells. The yield of progenitors from peripheral blood can be greatly increased by treatment of the donor with hematopoietic growth factors.37 Transplantation of growth-factor–mobilized peripheral blood stem cells from HLA-identical siblings has been associated with rapid hematological recovery,38-40 and it is possible that immune reconstitution might also be improved. To date, there has been no detectable increase in the risk of acute GVHD in patients receiving mobilized peripheral blood stem cells, despite a much larger number of donor T cells compared with marrow.38-40 The data reported here provide rationale for testing the hypothesis that the use of mobilized peripheral blood stem cells from unrelated volunteer donors may improve cure of leukemia by providing better reconstitution of hematopoietic and immune function.
Supported in part by National Institutes of Health Grants No. CA 15704, CA 18029, CA 18221, and A1 33484. J.S. is a recipient of Grant No. PF94 37727364 from the Secretarı́a de Estado de Universidades e Investigación, Ministerio de Educación y Ciencia and a grant from Hospital Clı́nic, Barcelona, Spain.
Address reprint requests to Claudio Anasetti, MD, Clinical Research Division, Fred Hutchinson Cancer Research Center, 1124 Columbia St, Seattle, WA 98104-2092.