Abstract
Hemophagocytic lymphohistiocytosis (HLH) is caused by the hyperactivation of T cells and macrophages. The clinical characteristics associated with this disease result from overproduction of Th1 cytokines including interferon-γ (IFN-γ), interleukin-2 (IL-2), and tumor necrosis factor-α (TNF-α). In this study, we analyzed the production of IL-12 and IL-4, which determine Th1 and Th2 response, respectively, and IL-10, which antagonizes Th1 cytokines, in 11 patients with HLH. IL-12 was detected in plasma in all patients (mean peak value, 30.0 ± 5.0 pg/mL), while IFN-γ was massively produced in nine patients (mean peak value, 79.2 ± 112.0 U/mL). IL-4 was not detected in any of the patients. Plasma IL10 levels were elevated in all patients (mean peak value, 2,698.0 ± 3,535.0 pg/mL). There was a positive correlation between the levels of IFN-γ and IL-10 (P < .01). The plasma concentrations of these cytokines were initially high, before decreasing after the acute phase. However, the decrease in IL-10 levels was slower than that of IFN-γ. Although the concentration of IL-12 was high at the acute phase, in some patients, a peak in the level was delayed until the chronic phase. Thus, in HLH, production of cytokines that promote development of Th1 cells appears to be predominant over that for Th2 cell development. Overproduction of IL-10 was also observed indicating that a mechanism suppressing hyperactivation of Th1 cells and monocytes/macrophages functions in patients with this disease.
HEMOPHAGOCYTIC lymphohistiocytosis (HLH), which affects otherwise healthy children, is characterized by hyperactivation of T cells and macrophages and is commonly triggered by viral infection.1-4 Patients with HLH show persistent high-grade fever, pancytopenia, coagulation abnormality, liver dysfunction, and proliferation of benign histiocytes with hemophagocytosis in the bone marrow, lymph nodes, spleen, and liver.1-4 These clinical characteristics result from the overproduction of cytokines, including interferon-γ (IFN-γ), interleukin-2 (IL-2), and tumor necrosis factor-α (TNF-α), by activated T cells (Th1 cells) and macrophages, which leads to a chain reaction.5-10
Th1 and Th2 cells were initially identified in the mouse and later in the human.11,12 Th1 and Th2 response is determined by the balance between IL-12 and IL-4.12-16 IL-12, mainly produced by antigen presenting cells such as B cells and macrophages, favors Th1 response and induces IFN-γ production from Th1 cells.14-16 IFN-γ enhances IL-12 production, helping to generate Th1 cells directly as well as promoting the differentiation of Th0 cells into Th1 cells.14-16 IL-4, one of the major Th2 cytokines, favors Th2 response. IL-10, which is produced from both IL-12–induced Th1 and IL-4–induced Th2 subsets,12 inhibits production of IL-12, as well as other Th1 cytokines including IFN-γ and IL-2.15-17 The suppressive effects of IL-10 result in inhibition of the functions mediated by T cells, monocytes/macrophages and natural killer (NK) cells.17-21 Furthermore, IL-10 suppresses the production of inflammatory cytokines and an anti-inflammatory agent, IL-1ra, as well as procoagulant activity.17-23
Thus, in addition to IFN-γ, IL-10, IL-4, and IL-12 are major cytokines determining Th1 and Th2 response and are supposed to play important roles in HLH.12 However, no study of these cytokines in HLH has been conducted. We measured the plasma levels of IFN-γ, IL-10, IL-4, and IL-12 in HLH patients to evaluate the roles of these cytokines in an immunologically hyperreactive state and to shed light on the immunological mechanism causing HLH.
MATERIALS AND METHODS
Patients.The plasma concentration of IFN-γ, IL-12, IL-4, and IL-10 was measured in 11 children with HLH, ranging in age from 8 months to 10 years. None of the children had any other diseases. All patients had a persistent high-grade fever, hepatosplenomegaly, liver dysfunction, pancytopenia, hyperferritinemia, and hypertriglyceridemia, while eight of the 11 patients had disseminated intravascular coagulopathy (DIC) (cases 1 through 8) (Table 1). All 11 children were diagnosed as having HLH based on clinical characteristics and hemophagocytosis by activated macrophages in the bone marrow. All patients were treated with immunosuppressive agents such as steroids and cyclosporine A (CsA). Cytotoxic agents including etoposide were administered in nine patients (cases 1 through 8, and 10) and plasma exchanges were done for seven patients (cases 1 through 7). Three patients had recurrent diseases (cases 1, 3, and 7). Four of the 11 patients (cases 1 through 4) died of uncontrollable disease.
Cytokine assay.Plasma IFN-γ levels were measured by an immunoradiometric assay (Medgenics Co, Fleurus, Belgium) (the limit of detection was 0.5 U/mL). Plasma IL-12, IL-4, and IL-10 levels were measured by sandwich enzyme-linked immunosorbent assay (ELISA). The ELISA kits and monoclonal antibodies (MoAbs) used were as follows: IL-12: the IL-12 heterodimer, p70, was measured using a rat MoAb, 20C2, and a polyclonal antibody, R7926 (the limit of detection was 5.0 pg/mL), IL-4: R & D Systems, Minneapolis, MN (the limit of detection was 3.0 pg/mL), IL-10: Biosource International, Camarillo, CA (the limit of detection was 5.0 pg/mL). The ELISA kit for IL-10 assay is specific for human IL-10 and not interfered by viral IL-10 produced by Epstein-Barr virus (EBV). Collected plasma samples were stored at −20°C until use.
RESULTS
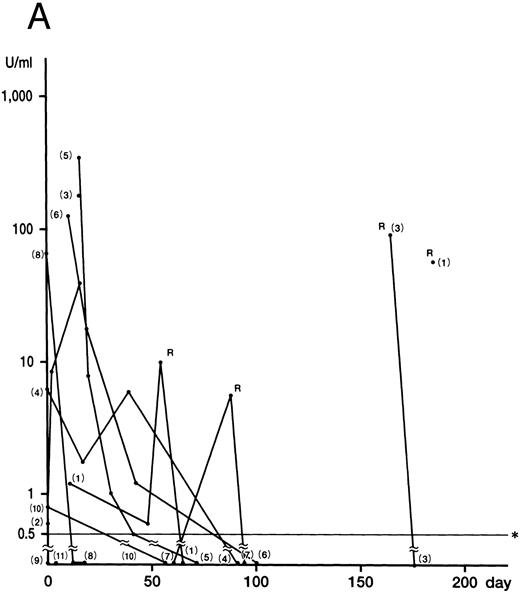
IFN-γ was detected in nine of 11 HLH patients at onset or in the early phase of the recurrent disease (cases 1 through 8, and 10). The mean of the peak values was 79.2 ± 112.0 U/mL (0.8 to 340.0 U/mL). Levels of IFN-γ decreased rapidly in all nine patients (
Fig 1. Plasma concentrations of IFN-γ (A), IL-12 (B), and IL-10 (C) in 11 patients with HLH. Numbers indicate case numbers. R indicates recurrence of the disease. Asterisks indicate the limit of detection.
Fig 1. Plasma concentrations of IFN-γ (A), IL-12 (B), and IL-10 (C) in 11 patients with HLH. Numbers indicate case numbers. R indicates recurrence of the disease. Asterisks indicate the limit of detection.
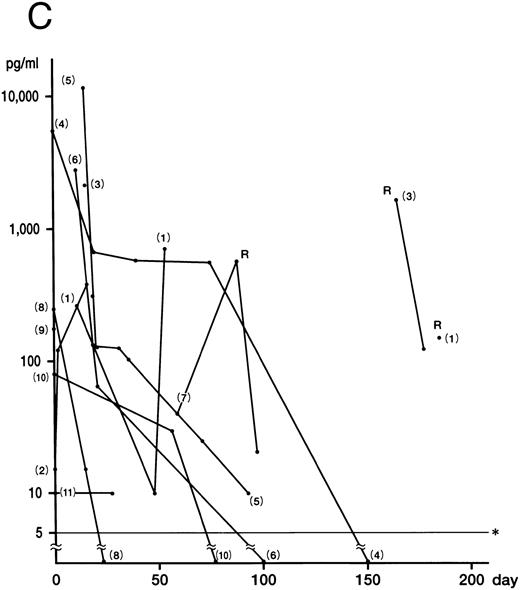
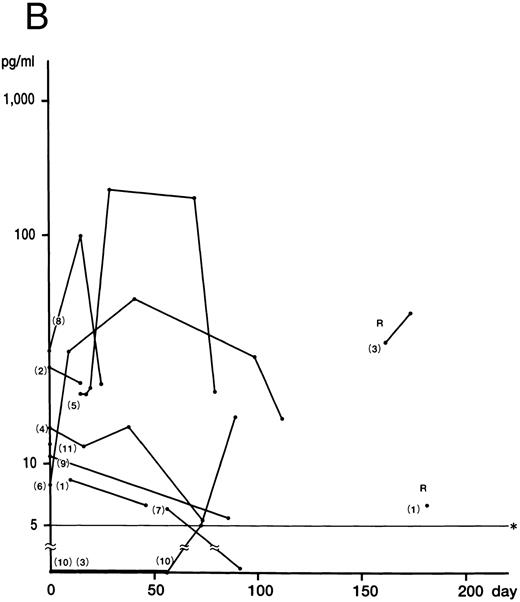
Fig 1A). IFN-γ was not detected in the remaining two patients (cases 9 and 11), who showed no DIC. Plasma IL-12 was detected at some stage of the disease in all patients (Fig 1B). The mean of the peak values was 30.0 ± 5.0 pg/mL (5.0 to 212.0 pg/mL). Of the six patients whose IFN-γ and IL-12 levels were serially measured, five showed a delayed peak in their IL-12 level, as compared with IFN-γ (cases 3, 5, 6, 8, and 10). The decrease in the IL-12 level in case 4 parallelled that of IFN-γ patients. The mean of the peak values of the IL-10 concentration was 2,698.0 ± 3,708.0 pg/mL (range, 10.0 to 14,800.0 pg/mL). In nine patients, the IL-10 plasma concentration was highest at the active phase of the disease and decreased to within the normal range after recovery from the illness (Fig 1C). Although the plasma levels of IL-10 correlated to those of IFN-γ (P < .01), IL-10 concentrations decreased more slowly than those of IFN-γ. IL-4 was undetectable throughout the clinical course of the disease in all patients.
Although no correlation between cytokine levels and patient prognosis was found, two cases (cases 9 and 11) of three patients without DIC (cases 9 through 11) showed no increase in IFN-γ levels, despite having increased IL-10 levels. In addition, case 10, with a slight increase in IFN-γ, displayed high IL-10 levels.
DISCUSSION
HLH is caused by a variety of infections and characterized by pancytopenia and histiomonocytic proliferation and phagocytosis in the bone marrow and the lymphoid system.1-4 It has been suggested that the pathogenesis of this syndrome is uncontrolled T-cell activation, which causes oversecretion of Th1 cytokines such as IFN-γ and IL-2, further activating T cells and monocytes/macrophages1-10,24 Most of clinical conditions including coagulopathy are considered to result from hypercytokinemia. Inflammatory cytokines such as IL-1, TNF-α, IL-6, and IL-8 produced from monocytes/macrophages activated by IFN-γ derived from T cells may induce endothelial cell procoagulant in HLH.23 This hypothesis is supported by the finding that patients without elevation of IFN-γ did not have serious symptoms or DIC.
In cases of HLH, it has been reported that IL-2, TNF-α, IL-6, macrophage colony-stimulating factor (M-CSF ), and IFN-γ plasma levels are elevated during the accelerated phase, returning to normal levels in remission.6,7,25 However, there has been no study of the cytokines that determine Th1 and Th2 response, or of those that antagonize Th1 cytokines in HLH. We measured plasma concentrations of IL-12 and IL-4, which stimulate the production of Th1 and Th2 cytokines, respectively,12 and IL-10, which suppresses Th1 cytokines.
IL-12, originally identified as a NK cell stimulatory factor produced by monocytes/macrophages, B cells, and other accessory cells in response to bacteria or bacterial products, favors Th1 response.14-16 The plasma concentrations of IL-12 were elevated in all patients. It seems possible that IL-12 was secreted by macrophages stimulated by IFN-γ. IL-12 may induce development of cells producing Th1 cytokines and also augment production of IFN-γ by these cells and NK cells, leading to further enhancement of IL-12 production by monocytes/macrophages, as noted in in vitro studies.15 16 While the production of IL-12 was rapid and observed in the acute phase, IL-12 levels maximized after the acute phase and the decline of IFN-γ plasma levels, in most patients.
In contrast, IL-4, which is produced by Th2 helper cells as well as IL-10, suppresses IL-10 secretion from macrophages and favors Th2 response,12 14 was not detected in any of the HLH patients. Although the optimal plasma concentrations of IL-4 for antagonizing Th1 response might be lower than the detectable level of our assay, it is likely that IL-4 does not play an important role in HLH, resulting in the predominance of Th1 cytokines over Th2.
IL-10, produced by monocytes/macrophages, activated B cells, and Th0 and Th2 cells in the mouse and Th1 and Th2 cells in the human, suppresses synthesis of Th1 cytokines including IFN-γ, IL-2, and TNF-α by Th1 cells and of IL-1, IL-6, IL-8, IL-12, and granulocyte-macrophage colony-stimulating factor (GM-CSF ) by monocytes/macrophages.12-14,17,18 In this study, massive production of IL-10 was found to correlate with the production of IFN-γ. Several reports have shown that the production of IL-10 increases during shock, suggesting that IL-10 may be involved in the control of the inflammatory response induced by bacterial products.26-29 In addition, IL-10 was shown to reduce the severity of the shock syndrome induced by anti-CD3 monoclonal antibody in mice.30 Therefore, it appears that overproduction of IL-10, seemingly by activated Th1 cells and macrophages, suppresses activated T cells and macrophages and antagonizes an immunologically hyperreactive condition induced by IFN-γ.
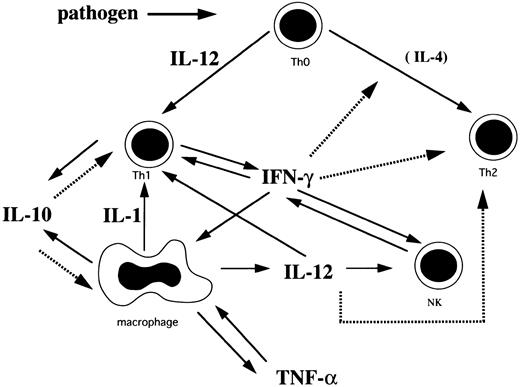
Based on the findings of this study and others, we have hypothesized the cytokine network in HLH (Fig 2). Th1 cells initially stimulated with pathogens secrete IL-2 and IFN-γ. IFN-γ stimulates macrophages to induce rapid production of IL-10, IL-12, IL-1, and TNF-α. IL-1 activates T cells and induces production of IL-2.21,23 31 Although IL-10 is produced by Th1 and Th2 cells and macrophages, it is probable that IL-10 is mainly produced by Th1 cells and macrophages because these cells are activated in HLH, while IL-4, a major Th2 cytokine, was not detected. IL-10 inhibits Th1 cells and the production of Th1 cytokines and negatively regulates the production of IL-10 from macrophages, resulting in a rapid decrease of plasma IFN-γ and IL-10 levels. IL-12 produced from macrophages activates NK cells and Th1 cells to produce IFN-γ. This stimulatory effect seems to persist even after the acute phase because of the sustained high plasma IL-12 level. However, this effect does not last, as IFN-γ production becomes exhausted. IL-12 also may promote differentiation of Th0 cells into Th1 cells and IFN-γ suppresses development of Th2 cells.
A plausible cytokine network mediated by Th1 cells, Th2 cells, macrophages, and NK cells in HLH. Solid arrows indicate stimulation or secretion and dotted arrows indicate inhibition.
A plausible cytokine network mediated by Th1 cells, Th2 cells, macrophages, and NK cells in HLH. Solid arrows indicate stimulation or secretion and dotted arrows indicate inhibition.
Thus, predominance of Th1 cytokines in HLH seems to be accelerated by overproducion of IL-12 probably produced from activated Th1 cells and macrophages, resulting in a chain reaction between Th1 cells and macrophages. In addition, an antagonizing mechanism against activated Th1 cells and macrophages also appears to function.
ACKNOWLEDGMENT
We thank S. Hamano and M. Fujiwara for typing the manuscript.
Supported in part by the grant-in-aid from the Ministry of Education, Science and Culture of Japan (Tokyo).
Address reprint requests to Yuko Osugi, MD, Department of Pediatrics, Osaka University Hospital; 2-2, Yamadaoka, Suita, 565, Japan.