Abstract
Patients with a lymphohematopoietic malignancy considered to be at high risk for posttransplant relapse were enrolled in a study to compare the use of cyclosporine (CSP) as a single agent with a combination of methylprednisolone (MP) and CSP for graft-versus-host disease (GVHD) prophylaxis after marrow transplantation from an HLA-identical sibling donor. Sixty patients were randomized to receive CSP only and 62 were randomized to receive CSP plus MP. Daily CSP was started on day −1 (5 mg/kg/d intravenously) and administered at gradually reduced doses until day 180. MP was started on day 7 at 0.5 mg/kg/d, increased to 1.0 mg/kg/d on day 15, started on a taper schedule on day 29, and discontinued on day 72. All 104 evaluable patients (surviving ≥28 days) had sustained engraftment. The incidence rates of grades II-IV acute GVHD were 73% and 60% for patients receiving CSP and CSP plus MP, respectively (P = .01). No difference was seen for grades III-IV GVHD. However, chronic GVHD occurred somewhat more frequently in patients receiving CSP plus MP (44%) than in patients receiving only CSP (21%; P = .02). The incidence of de novo chronic GVHD was marginally higher in patients receiving CSP plus MP (P = .08). No significant differences in the risk of infections were observed. There was a suggestion that the risk of relapse was lower in patients receiving CSP plus MP (P = .10) and, although the overall survival in the two groups was not different (P = .44), there was a slight advantage in favor of CSP plus MP-treated patients for relapse-free survival (P = .07). These results suggest that prophylactic MP, when combined with CSP, has only limited efficacy in acute GVHD prevention and may increase the probability of chronic GVHD.
CYCLOSPORINE (CSP) HAS been used clinically for almost 2 decades. Although introduced with high expectations into the practice of marrow transplantation, the efficacy of CSP as a single agent for the prevention of graft-versus-host disease (GVHD) was not superior to that of a then standard regimen of intermittent methotrexate (MTX).1-3 However, second generation studies showed a significant reduction in the incidence of acute GVHD and improved survival when CSP was administered in combination with MTX.4-6 One disadvantage of the combined regimen was that the myelosuppressive effect of MTX delayed hematopoietic recovery as compared with results with CSP alone.4,5 In addition, it has been suggested that prevention of acute GVHD may be associated with an increased probability of leukemic relapse.7 Other investigators combined CSP plus prednisone.8,9 This combination allowed for more rapid hematopoietic recovery compared with MTX-containing regimens, albeit at the price of a higher incidence of GVHD than seen with MTX plus CSP.5,6 Also, evidence has been presented that the addition of prednisone may increase the risk of infection,10 although this was not the case in an earlier study.11 The combination of CSP plus prednisone has never been compared in a prospective randomized study to single-agent CSP. Such a study is of interest for several reasons. (1) Although the addition of methylprednisolone (MP) to CSP may decrease the incidence of GVHD, it may add to toxicity and, as a result, fail to improve overall outcome.12 (2) Earlier noncontrolled studies suggested that the use of MP increased the probability of developing chronic GVHD.12,13 (3) At least one study comparing CSP plus MTX to CSP combined with MTX plus MP showed a decreased incidence of relapse with the incorporation of MP.12 Therefore, we performed a prospective randomized study comparing a combination of CSP plus MP with single-agent CSP for GVHD prophylaxis in patients considered at high risk of recurrent malignancy posttransplant.
MATERIALS AND METHODS
Patients
From September 1991 through July 1994, 123 patients considered at high risk for posttransplant relapse were registered on this protocol. This included patients with lymphoid and myeloid malignancies who were not in remission (relapse or resistant disease) and with lymphoid malignancies in third or subsequent remission or with myeloid leukemia in second or subsequent remission. One patient (unique patient number 7559) declined a transplant after randomization. Characteristics of the remaining 122 patients (followed for 17.5 to 59 [median, 41] months) are shown in Table 1. Seven stratification variables were considered: diagnosis (myeloid v lymphoid), disease stage (remission v relapse or accelerated phase), age (<25 years v ≥25 years), donor/patient gender, high risk v low risk for posttransplant relapse,14 conditioning regimen (1,200 cGy total body irradiation [TBI] or none v >1,200 cGy TBI), receiving intravenous (IV) Ig (yes v no), and treatment in laminar air flow room (yes v no). As shown in Table 1, the study arms were balanced with respect to these risk factors. Protocol and consent forms were approved by the Institutional Review Board of the Fred Hutchinson Cancer Research Center (Seattle, WA). Risks and benefits of treatment regimens were explained to each patient in detail before hospital admission.
Donor Selection
All donors were HLA-genotypically identical relatives. Serological (class I) and molecular typing (class II) were performed according to standard techniques.
Conditioning Regimens and Transplantation
Conditioning regimens are summarized in Table 1. Fractionated irradiation was delivered from two opposing 60Co sources at an exposure rate of 7 cGy/min. Within 4 hours of the last TBI exposure or 36 hours after the last dose of chemotherapy, donor marrow was infused IV. The marrow cell dose ranged from 0.5 to 6.1 (median, 1.8) × 108 cells/kg. The day of marrow infusion was designated day 0. Engraftment was defined as the first of at least 3 consecutive days on which the neutrophil count surpassed 0.5 × 109/L after the posttransplant nadir.
GVHD Prophylaxis, Assessment, and Treatment
All patients received CSP and, in addition, were randomized to receive or not to receive MP in a nonblinded fashion; regimens for both drugs were identical to those previously described by others.8,15 CSP was administered at doses of 5 mg/kg/d as a continuous IV infusion on days −1 through 3 and at 3 mg/kg/d on days 4 through 14. On days 15 through 35, 3.75 mg/kg/d was administered IV; patients who were discharged to the Outpatient Department received oral CSP at a dose of 5 mg/kg twice daily. This dose was continued through day 83 if no toxicity developed; it was tapered to 4 mg/kg on day 84, to 3 mg/kg on day 98, to 2 mg/kg twice a day on day 120, and continued through day 180. Downward dose adjustments were made if renal toxicity developed. MP was started on day 7 and administered through day 14 at a dose of 0.25 mg/kg IV twice daily. On day 15, the MP dose was increased to 0.5 mg/kg twice daily and then decreased again to 0.25 mg/kg orally on day 29, to 0.15 mg/kg on day 43, and to 0.1 mg/kg on day 57 through day 72, when treatment was stopped.
Assessment, grading, and treatment of acute GVHD have been reported previously.16-19 Acute GVHD was treated by increasing the dose of MP to 2 mg/kg in patients who had been randomized to CSP plus MP or by instituting MP in patients randomized to receive CSP only. The plan was to treat patients for 14 days at the full dose and then begin to taper steroids. Patients who did not respond to MP as primary therapy were generally treated with antithymocyte globulin.
Patients were evaluated for the presence of chronic GVHD before discharge from the center as described.17,20 21 Studies included hematopoietic and chemical parameters, skin and lip biopsies, Schirmer's test, pulmonary function tests, and other examinations as indicated. Patients with clinical extensive chronic GVHD were treated with continued immunosuppression. Patients with subclinical disease or without evidence of GVHD were observed at regular intervals, and therapy was instituted if clinical disease developed.
Other Supportive Care
All patients received prophylactic systemic antiviral and antibacterial antibiotics and trimethroprim-sulfamethoxazole for Pneumocystis carinii prophylaxis as described.5 Twelve patients were placed in a laminar air flow room for protective isolation. Ten patients received intermittent IVIg as part of a concurrent study. In the remaining patients, IVIg was administered only when serum IgG levels decreased to less than 400 mg/dL.
Infections
The infection data for this analysis were collected prospectively on coded data sheets for the time interval from day 0 through day 100 and categorized in a blinded fashion (M.B.) as described.10,22 Briefly, bacteremia was defined as one or more positive blood cultures with any bacterial organism regardless of associated symptoms. Any culture record for a given organism within 21 days of an initial positive blood culture for that organism was considered to represent the same infection and was not considered to indicate a new bacteremia. Blood culture records for a different organism occurring any time after a positive culture for another organism was considered to be a separate bacteremia. Blood culture records for multiple organisms on the same day were considered to be a single polymicrobial bacteremia. Culture records for a micrococcus or non-JK corynebacterium species or aerobic diphtheroids were not included in the present analysis because they were considered to be contaminants. Organ site infections were identified as positive bacterial cultures from normally sterile sites (eg, sinuses). Fungemia was defined as occurrence of one or more positive blood cultures with any fungal organism regardless of associated symptoms. Invasive mold infections were defined as biopsy-proven tissue invasion or positive cultures as described.22 Analyses were performed considering all infections, bacterial infections, fungal infections, and combined invasive fungal, gram-negative, and polymicrobial infections.
Statistical Considerations
Design.The primary response variable in the study design was the incidence of grades II-IV acute GVHD. Secondary responses to be analyzed included the development of chronic GVHD, incidence of infections, relapse, and survival. The CSP arm was expected to show a 50% incidence of grades II-IV GVHD. For purposes of this study, reduction to 25% would have been considered clinically significant. For a test with .05 statistical significance and with power 90% a maximum sample size of 92 patients per arm would be required.23 An interim analysis was performed as planned upon enrollment of 71 patients. The estimated incidence rates of acute GVHD for patients receiving CSP or CSP plus MP were 60% and 44%, respectively, yielding a one-sided P value of .092. These interim results suggested a beneficial effect of the drug combination but were not strong enough to terminate the study early. Patient enrollment was therefore continued, but the goal of accruing 92 patients had to be abandoned as a consequence of competing clinical protocols.
Analysis.Log-rank test statistics and Cox models were used in an analysis by intent to treat for time to event data including acute and chronic GVHD, survival, relapse, nonrelapse mortality, relapse-free survival, and infection. A second analysis was performed by actual treatment, excluding 8 patients in the CSP arm and 7 patients in the CSP plus MP arm who failed to receive the prescribed treatment based on decisions by the attending physician. For additional analysis of the infection data, the Anderson-Gill counting process model24 was used, which is an extension of the Cox model that accommodates multiple events (infection episodes) in the same individual. Cumulative incidence and conditional probability estimates25 26 were used in the analysis of acute and chronic GVHD. Kaplan-Meier estimates are presented for disease-free survival. Software package S-plus 3.3 (Mathsoft Inc, Seattle, WA) was used for the Anderson-Gill model, and SAS (SAS Institute Inc, Cary, NC), Stata (Stata Statistical Software: Release 5.0; Stata Corporation, College Station, TX), or Gauss (version 3.2.6; Aptech Systems Inc, Maple Valley, WA) were used for all other analyses. Results were analyzed as of January 1, 1996.
RESULTS
Engraftment
All 104 patients surviving more than 28 days had sustained engraftment; 18 patients who died before day 28 (12 and 6 patients on CSP and CSP plus MP, respectively) were considered unevaluable for engraftment.
GVHD
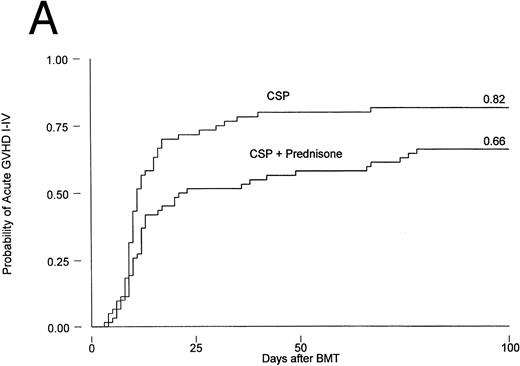
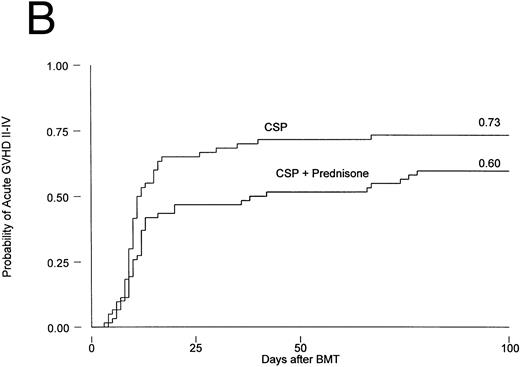
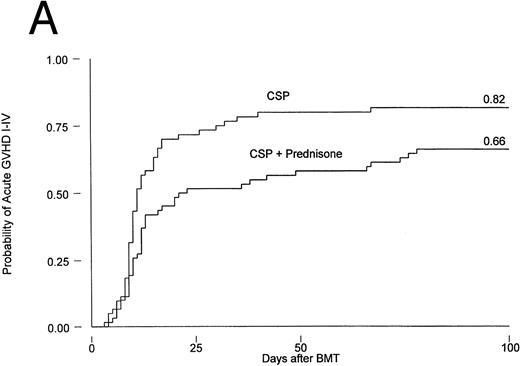
Results are summarized in Tables 2 and 3 and Figs 1 and 2. The cumulative incidence of grades I-IV acute GVHD was 82% and 66% for patients on CSP and CSP plus MP, respectively (P = .001, log-rank test). Grades II-IV acute GVHD, the primary endpoint of the study, developed in 44 patients (73%) on the CSP arm at 3 to 67 (median, 10) days after transplantation, compared with 37 patients (60%) on the CSP plus MP arm at 4 to 78 (median, 12) days after transplantation. Acute GVHD, grades III-IV, developed in 24 patients (40%) receiving CSP and 21 patients (34%) receiving CSP plus MP (Table 2A). Although the incidence of acute GVHD in patients receiving only CSP was higher in all target organs, the difference was most striking in the skin (Table 2B). In Cox regression analysis (Table 3), the risk of developing acute GVHD among patients on CSP was significantly higher than for patients on CSP plus MP for any grade (P = .001) and for grades II-IV (P = .01) but not for severity grades III-IV (P = .28). As shown in Table 3, results were basically the same in the analysis by actual treatment. The decision not to administer the GVHD prophylaxis prescribed by randomization was made by the attending physician. Reasons included mainly concern about steroid administration to patients who were infected or suboptimal prophylaxis with CSP as the only drug.
Probability of acute GVHD in patients receiving GVHD prophylaxis with CSP alone or CSP plus MP. (A) Acute GVHD grades I-IV (P = .001); (B) acute GVHD grades II-IV (P = .01).
Probability of acute GVHD in patients receiving GVHD prophylaxis with CSP alone or CSP plus MP. (A) Acute GVHD grades I-IV (P = .001); (B) acute GVHD grades II-IV (P = .01).
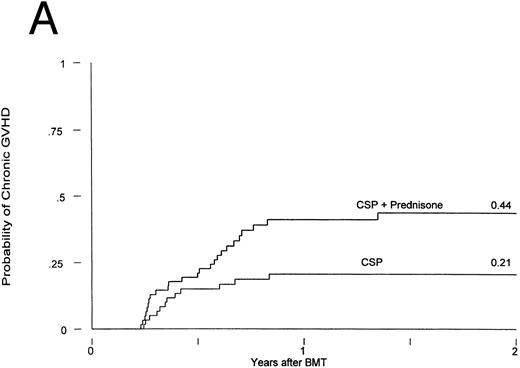
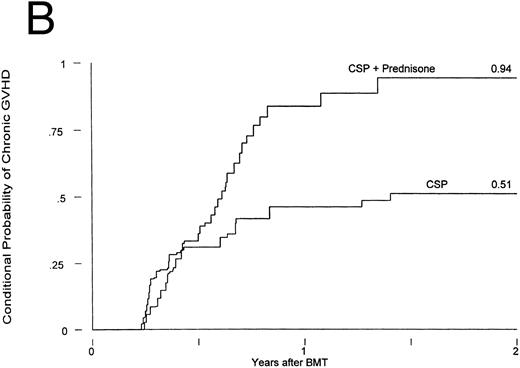
Chronic GVHD in patients receiving GVHD prophylaxis with CSP alone or CSP plus MP. (A) Probability (P = .02); (B) conditional probability (P = .03).
Chronic GVHD in patients receiving GVHD prophylaxis with CSP alone or CSP plus MP. (A) Probability (P = .02); (B) conditional probability (P = .03).
Chronic GVHD developed in 12 of the patients receiving CSP at 75 to 305 (median, 127) days posttransplant and in 25 of the patients receiving CSP plus MP at 75 to 492 (median, 182) days posttransplant. Thus, the cumulative incidences were 21% and 44% for patients receiving CSP and CSP plus MP, respectively (Fig 2A). The relative risk of developing chronic GVHD for patients receiving CSP plus MP was 2.33 (confidence interval [CI], 1.16, 4.71; P = .02). The conditional probabilities (conditional on surviving) of developing chronic GVHD for the two groups were 51% and 94%, respectively (P = .03; Fig 2B). De novo chronic GVHD developed in 1 of 8 patients at risk on CSP (these were the only 8 patients who never received any MP) and in 12 of 19 patients at risk on CSP plus MP, respectively; the relative risk was 6.28 (CI, 0.81, 48.4; P = .08). The pattern was the same in the analysis by actual treatment, although the differences did not reach significance.
Infections
The incidence of clinically relevant infections is summarized in Table 3. Whereas the numbers of infectious events, particularly fungal infections, appeared to be slightly higher in the group of patients receiving CSP plus MP, none of the differences was statistically significant. This was true for both the number of patients experiencing infections (first infection) and the number of episodes (all infections). Because treatment of GVHD involved the use of MP, it was possible that the therapeutic use of MP in patients originally randomized to receive CSP only would obscure differences between the two prophylactic groups. Therefore, an additional analysis was performed with censoring of patients at the time of treatment for acute GVHD. By that time, 14 patients on the CSP arm and 20 patients the CSP plus MP arm had developed an infection; this difference was not significant (relative risk, 0.99; CI, 0.49, 2.0; P = .98). Similarly, in the analysis by actual treatment no significant difference was observed.
Relapse of the Underlying Disease
Among CSP-treated patients, 17 had a recurrence of their underlying disease compared with 13 receiving CSP plus MP prophylaxis (not significant). Relapse tended to occur later in patients receiving CSP plus MP, but this difference was not significant (P = .10).
Survival
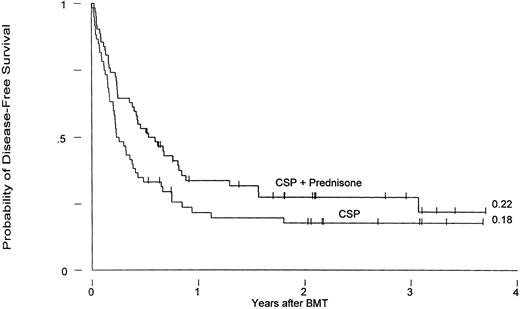
Currently, 36 patients are surviving, 17 who had received CSP and 19 CSP plus MP prophylaxis, for Kaplan-Meier survival estimates at 3 years of 26% and 23%, respectively (P = .45). Three-year relapse-free survival estimates for the two groups are 18% and 22%, respectively (P = .07; Fig 3).
Leukemia-free survival in patients receiving GVHD prophylaxis with CSP alone or CSP plus MP (P = .07).
Leukemia-free survival in patients receiving GVHD prophylaxis with CSP alone or CSP plus MP (P = .07).
Causes of death are listed in Table 4. There was no difference in overall mortality between the two groups and there was no obvious difference in regard to any particular cause of death.
DISCUSSION
MP has been used extensively in patients undergoing marrow or solid organ transplantation and is considered standard therapy for treatment of established acute and chronic GVHD.27-30 Several studies have also incorporated MP for GVHD prophylaxis, but its role for this indication is controversial.8,9,15,31-33 A recent study suggested that the use of MP concurrently with MTX and CSP increased — rather than decreased — the incidence of GVHD, leading to the speculation that MP interfered with the antimetabolite MTX, thereby reducing or neutralizing its immunosuppressive effect.12 However, it is interesting that, in the same study, patients who had received MP were somewhat less likely to suffer a posttransplant relapse than patients not receiving MP.12 The objective of the present trial was to compare in a prospective, randomized study single-agent CSP and CSP plus MP in regard to GVHD prevention and incidence of infection. As the study population, we chose patients with myeloid or lymphoid malignancies who were considered to be at high risk of disease recurrence after transplantation. Because these patients would be expected to potentially benefit from a graft-versus-leukemia effect,34-37 not using the standard regimen of CSP plus MTX and accepting a possibly higher incidence of GVHD was felt to be acceptable.
As suggested by earlier noncontrolled studies,15 the incidence of acute GVHD of all severity grades in the present study, although high overall, was lower in patients receiving a combination of CSP plus MP. However, this difference in overall grading was significant only for mild to moderate but not for severe (grades III-IV) manifestations of GVHD. The difference was most striking in the skin, consistent with earlier observations that skin manifestations are particularly responsive to steroids.30 Of note was the rather early onset of skin exanthemas thought to represent GVHD in some patients. Although it is not possible to exclude the possibility of nonspecific rashes, the fact that they occurred in both treatment arms suggests that the omission of MTX, currently used in most standard regimens,5,6 contributed to this phenomenon. This notion is supported by observations in earlier randomized studies comparing MTX and CSP.1 2
GVHD prophylaxis also influenced the development of chronic GVHD, albeit in a direction opposite to that observed with acute GVHD: the incidence of chronic GVHD was higher in CSP plus MP-treated patients than among patients receiving CSP only. A difference was still present if patients who developed acute GVHD and, therefore, received therapeutic MP even if originally randomized to receive CSP only were censored. The occurrence of de novo chronic GVHD was marginally more likely in patients on CSP plus MP prophylaxis (P = .08). The reason for such an effect of MP is not clear. Conceivably, MP, although suppressing an acute GVHD reaction, interfered with signals required for T-cell selection and the establishment of tolerance.38 As a result, host-reactive T cells would initiate GVHD once immunosuppression was tapered or discontinued. Although it is known that acute GVHD represents a major risk factor for the subsequent development of chronic GVHD,39 40 the present results suggest that the prophylactic use of MP, even though effective in reducing the incidence of acute GVHD, does not provide prophylaxis for chronic GVHD.
A previous prospective trial in patients receiving MTX plus CSP and randomized to receive or not to receive MP as GVHD prophylaxis had shown a significantly higher probability of infection in patients on MP.10 In the present study, the probabilities of fungal and bacterial infections were similar in the two groups. There may be several reasons for the discrepancy between previous and present results. For one, the MP dose in the present study was lower (0.5 mg/kg/d v 1 mg/kg/d) in the early posttransplant period. Secondly, the use of MTX in the previous (but not in the current) study might impair mucosal integrity, thereby facilitating the development of invasive infections, an effect that may have been amplified by the addition of MP. The lack of a significant difference between CSP and CSP plus MP-treated patients in the present study was not explained by the therapeutic use of MP: results were indistinguishable (P = .98) when patients receiving therapeutic MP were censored.
The probability of relapse of the underlying disease is influenced not only by disease stage, but also by other factors, including the type and intensity of the GVHD prophylactic regimen and the development of acute or chronic GVHD.34-36 There was a suggestion in the present analysis that patients receiving CSP plus MP had a lower incidence of leukemic relapse (P = .10) and that relapse-free survival was better in the CSP plus MP group than among patients receiving CSP only (P = .07). These results are consistent with findings of an earlier trial showing that the addition of MP to CSP plus MTX resulted in fewer relapses.11 In that study we speculated that MP interfered with the efficacy of MTX and that the resulting increase in GVHD was associated with a more potent graft-versus-leukemia effect. However, in the present study, the incidence of acute GVHD was actually lower in the CSP plus MP group; the incidence of chronic GVHD was nevertheless increased as compared with patients receiving CSP alone. These data are consistent with either a direct antileukemic effect of MP or a graft-versus-leukemia effect associated with chronic GVHD.36 37
In conclusion, this randomized prospective study shows that a combination of CSP plus MP is more effective in preventing acute GVHD than CSP alone. The incidence of chronic GVHD, on the other hand, was higher in the CSP plus MP group, a finding in support of the notion that MP is not an effective agent for the prevention of chronic GVHD. However, only very few patients were able to avoid the use of MP completely. There was a suggestion that the prophylactic use of MP resulted in a slight improvement of relapse-free survival.
ACKNOWLEDGMENT
We thank all of the nurses and physicians on the Transplant Wards, the Outpatient Department, and the Long-term Follow-up Office for their contributions and Bonnie Larson and Harriet Childs for typing the manuscript. Special thanks to Russ Schwartz and the staff of the Microbiology Laboratory for compiling the bacterial and fungal culture data.
Supported by Public Health Service Grants No. CA15704, CA18029, CA18221, and HL36444 from the National Institutes of Health, Department of Health and Human Services, Bethesda, MD.
Address reprint requests to H. Joachim Deeg, MD, Fred Hutchinson Cancer Research Center, 1124 Columbia St, M318, Seattle, WA 98104-2092.