Abstract
The recently cloned cytokine interleukin-15 (IL-15) shares several functional activities with IL-2 in different cell systems. Although IL-15 does not show sequence homology with IL-2, it uses components of the IL-2 receptor (IL-2R) for binding and signal transduction, namely, p75 (β) and the p64 (γ) chains of IL-2R. To evaluate whether IL-15 is involved in the activation of granular lymphocytes (GL) in patients with lymphoproliferative disease of granular lymphocytes (LDGL), we evaluated the ability of IL-15 to stimulate GL proliferation, cytotoxic function, and the role of IL-2R β and γ molecules on relevant cells. Our results show that IL-15 stimulates cell proliferation and cytotoxic activity of GL in LDGL patients. Reverse-transcriptase polymerase chain reaction (RT-PCR) and phenotypic analyses using the anti–IL-2R γ-chain–specific TUGh4 monoclonal antibody (MoAb) indicate that both CD3+ and CD3− GL express the p64 IL-2R, a result previously unknown. IL-15 activity was inhibited by antibodies against p75 and p64 IL-2R chains, while no inhibitory effects are detectable with anti-p55 IL-2R antibody. The association of anti-p75 and anti-p64 IL-2R MoAbs resulted in a nearly complete (95%) inhibition of IL-15–induced GL proliferation. Using RT-PCR analysis, we demonstrated that highly purified CD3+ and CD3− GL did not express mRNA for IL-15 or IL-2. By contrast, a clear-cut IL-15 mRNA signal was detected by RT-PCR in patients' peripheral blood mononuclear cells, with monocytes likely accounting for the source of IL-15 in LDGL patients. However, even in concentrated supernatants from enriched monocyte populations, we could not demonstrate the presence of IL-15 protein. Using anti–IL-15 specific MoAbs, a membrane-bound form of this cytokine was demonstrated both on CD3+ and CD3− LDGL cells. By RT-PCR analysis, purified GL from these patients were found to express the message for IL-15 receptor α chain. Taken together, these results indicate that both CD3+ and CD3− GL are stimulated by IL-15 and that this cytokine mediates its activity through the β and γ chains of the IL-2R, providing further suggestions for the interpretation of the mechanisms that lead to cell expansion in patients with LDGL.
GRANULAR LYMPHOCYTES (GL) account for less than 15% of the normal lymphoid peripheral blood population. The large majority of these cells belong to the natural-killer (NK) subset, characterized by the CD3−CD16+CD56+ phenotype.1 A small proportion of GL, usually less than 3% in healthy subjects, expresses the CD3+CD16+ phenotype. A disorder recognized as lymphoproliferative disease of granular lympocytes (LDGL) is characterized by the marked and persistent increase in GL.2-4 Proliferating GL in patients with LDGL largely belong to the CD3+CD16+ T-cell subset (85%), usually expressing T-cell receptor (TCR) monoclonal rearrangements. The cases sustained by CD3− NK cells are less frequent (15%) and are mostly represented by polyclonal proliferations.5,6 A distinctive feature of both CD3+ and CD3− GL in LDGL patients is the expression of the β chain of the interleukin-2 (IL-2) receptor (IL-2R), which is not associated with the IL-2R α chain.7-9 We and others demonstrated that the IL-2R β chain is involved in the mechanisms that lead to proliferative and cytotoxic activities of GL in patients with LDGL.7-9 So far, no information is available on the expression of the γ chain of the IL-2R.
A novel cytokine, designated IL-15, has recently been identified in culture supernatants of a monkey kidney epithelial cell line, CV-1/EBNA.10-13 Although IL-15 shows no homology with IL-2, it shares some biologic effects with IL-2. In particular, IL-15 stimulates the proliferation of cytotoxic T-lymphocyte cell lines and phytohemagglutinin (PHA)-activated peripheral blood lymphocytes, induces activation of NK cells and lymphokine-activated killer activity, and promotes the proliferation and differentiation of preactivated normal B cells.11,14,15 IL-15 has been reported to use components of the IL-2R system for binding and signalling, ie, the β and γ chains.12 As for the cell source of IL-15, Northern analysis of human cells and tissues showed that IL-15 mRNA was most abundant in placenta and skeletal muscle, with detectable levels in heart, lung, liver, and kidney.10 In striking contrast to IL-2, IL-15 is not produced by T cells, whereas it can be released by monocyte/macrophage cells.16
The aim of the present study was to investigate the role of IL-15 in the activation pathways and the receptors involved in the expansion of GL in LDGL patients. In particular, the specific role of IL-15 and its relationship to the IL-2–induced proliferation and cytotoxicity were taken into account. By reverse-transcriptase polymerase chain reaction (RT-PCR) and flow cytometry analyses, we investigated whether proliferating GL of LDGL patients expressed the IL-2R γ chain. The contribution of α, β, and γ IL-2R on GL proliferation was also studied using specific antibodies. Our data indicate that IL-15 is able to activate both CD3+ and CD3− GL populations and cooperate with IL-2 to stimulate cell proliferation and cytotoxic activity. By RT-PCR, we demonstrated that both CD3+ and CD3− GL express the mRNA for the α chain of IL-15R.
MATERIALS AND METHODS
Patients.Fourteen patients (nine men and five women; mean age, 54 ± 12 years) were studied. In all cases, a chronic lymphocytosis (lasting > 6 months) sustained by at least 2,000 GL/μL was present in the peripheral blood.17 At the time of the study, none of the patients had received treatment, with the only exception being patient no. 4, who had received substitutive therapy for hemolytic anemia. Relevant immunologic data of cases under study are shown in Table 1.
Isolation of granular lymphocytes and monocytes. Peripheral blood mononuclear cells (PBMC) were obtained by Ficoll-Hypaque gradient centrifugation. For each patient, two aliquots of plasma were collected at the same time. The cell suspension was initially depleted of adherent cells by incubation for 45 minutes in plastic Petri dishes at 37°C in an atmosphere of 95% air and 5% CO2 . To remove the adherent monocytes, plastic dishes were sprayed with cold RPMI using a 20-mL syringe equipped with a 26-gauge needle. Cell populations were always found to contain greater than 90% CD14+ cells by flow cytometry.
The samples from patients under study were enriched for T-cell and NK GL using a modified version of the method previously described.18 GL were purified by a two-step sequential depletion using magnetic separations over columns (Mini MACS; Miltenvi Biotech, Bergisch Gladbach, Germany), as previously described.18 Briefly, for isolation of CD3+ GL, the cell suspension obtained as indicated earlier was incubated for 45 minutes at 4°C with anti-CD19 (Leu-12; Becton Dickinson, Sunnyvale, CA) monoclonal antibody (MoAb), anti-CD14 (Leu-M1; Becton Dickinson), and anti-CD4 (OKT4; Ortho, Raritan, NJ) MoAbs (patients no. 1 to 7) or anti-CD4 and anti-CD8 (OKT8; Ortho) MoAbs (patient no. 9); to obtain CD3− NK GL (cases no. 10 to 14), cells were incubated with anti-CD3 (OKT3; Ortho), anti-CD14, and anti-CD19 MoAbs. The cells rosetting with the antibody-coated beads were then isolated and removed by applying a magnetic system on the outer wall of the columns. Following this multistep negative-selection procedure, more than 97% of the cells were viable when tested by the trypan blue exclusion test and 92% to 97% showed GL morphology. Since monocytes have been reported to be a source for IL-15, particular care was taken to rule out contamination from these cell populations. Normal E+-rosetted peripheral blood cells were obtained from four different normal subjects using neuraminidase-treated sheep erhytrocytes, as previously reported.18
Cytokines and MoAbs.Recombinant IL-15 (specific activity, 3.33 × 105 U/mg) was kindly provided by Dr A. Troutt (Immunex, Seattle, WA). Recombinant human IL-2 (specific activity, 3.10 × 106 U/mg) was obtained from Biogen (Cambridge, MA). A panel of fluorescein isothiocyanate- and phycoerythrin-conjugated MoAbs, including anti-CD3 (Leu-4), CD4 (Leu-3), CD8 (Leu-2), CD16 (Leu-11c), CD56 (Leu-19), CD57 (Leu-7), CD25 (anti–IL-2R), and CD122 (anti–IL-2R p75), was obtained from Becton Dickinson. Azide-free anti-CD3 (OKT-3), anti-CD4 (OKT-4), and anti-CD8 (OKT-8) were obtained from Ortho Diagnostic. Anti-CD16 KD1 MoAb was kindly provided by Drs L. Moretta and A. Moretta (Genova, Italy).19 Purified anti-CD25 (B-B10 ) MoAb, which binds and blocks the p55 chain of IL-2R, was a gift of Dr J. Wijdenes (Besançon, France); ascites anti-p75 IL-2R TU27 MoAb (immunoglobulin G1 [IgG1]), which binds and blocks the p75 chain of IL-2R, and ascites anti-p64 TUGh4 (rat IgG2b), which binds and blocks the p64 chain of IL-2R, were kindly provided by Dr K. Sugamura (Tohoku, Japan) and Dr J. Hamuro (Kawasaki, Japan).20 Mouse M110 anti–IL-15 MoAb was kindly provided by Dr A. Troutt, Immunex; mouse M111 and M112 anti–IL-15 MoAbs were obtained from Genzyme (Boston, MA); and polyclonal rabbit anti–IL-15 was obtained from Pepro-Tech (Rocky Hill, NJ). For immunofluorescence analysis, control IgG1 and IgG2a and IgG2b were obtained from Becton-Dickinson; control rat antiserum consisted of ascites containing an irrelevant rat IgG2b MoAb (kindly provided by A. Rosato, Padova, Italy); control rabbit antiserum consisted of rabbit IgG myeloma (purified protein) purchased from Serotec (Oxford, UK); and goat anti-rabbit IgG and goat F(ab́)2 anti-rat IgG were obtained from Immunotech (Marseille, France).
Proliferative activity.GL from patients at the concentration of 1 × 106 cells/mL were cultured in 96-well round-bottom plates (Corning, New York, NY) for 72 hours at 37°C in 5% CO2 atmosphere with IL-15 (0.1, 1, 10, and 100 ng/mL), rIL-2 (0.1, 1, 10, and 100 ng/mL), anti-p75 IL-2R TU27 MoAb (10 μg/mL final dilution), anti-p55 IL-2R BB-10 MoAb (10 μg/mL final dilution), TUGh4 (10 μg/mL final dilution), or control isotype-matched IgG were added at the beginning of the culture. Each experiment was performed in quadruplicate. For the last 18 hours of culture, plates were pulsed with 1 μCi/well of (3H)thymidine (6.7 Ci/mmol; Du Pont, NEN, Boston, MA); cells were then harvested and the radioactivity measured with a β counter. Results are expressed as counts per minutes (cpm) ± SEM.
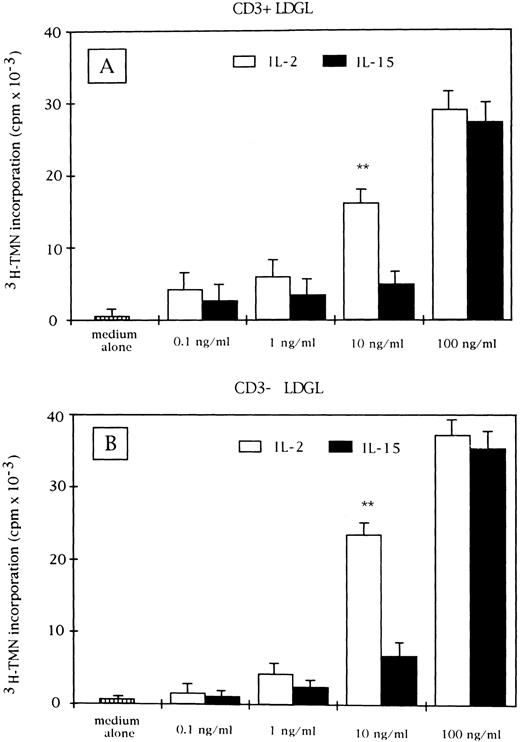
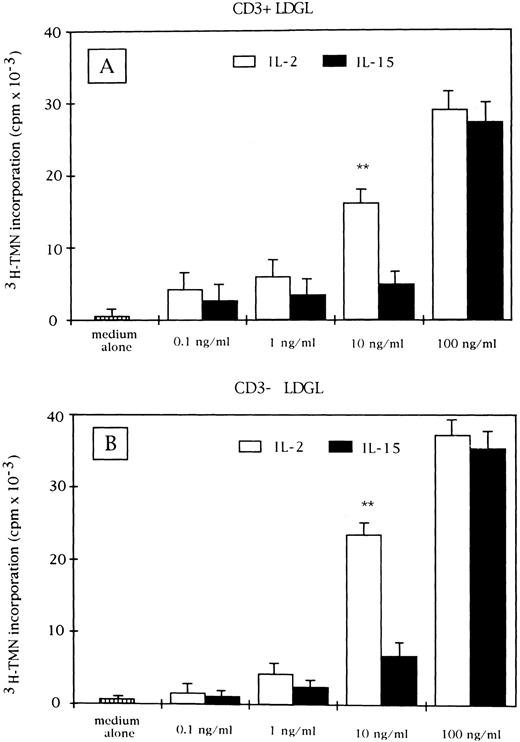
Proliferative activity induced by IL-15 (▪) and IL-2 (□) on CD3+ (A) and CD3− GL (B) cells derived from patients with LDGL (9 CD3+ and 5 CD3−). Data are expressed as cpm × 10−3 ± SEM. **P < .05.
Proliferative activity induced by IL-15 (▪) and IL-2 (□) on CD3+ (A) and CD3− GL (B) cells derived from patients with LDGL (9 CD3+ and 5 CD3−). Data are expressed as cpm × 10−3 ± SEM. **P < .05.
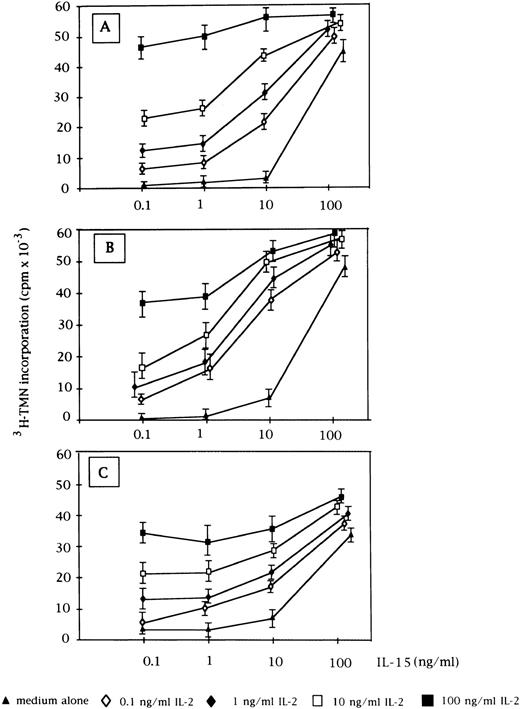
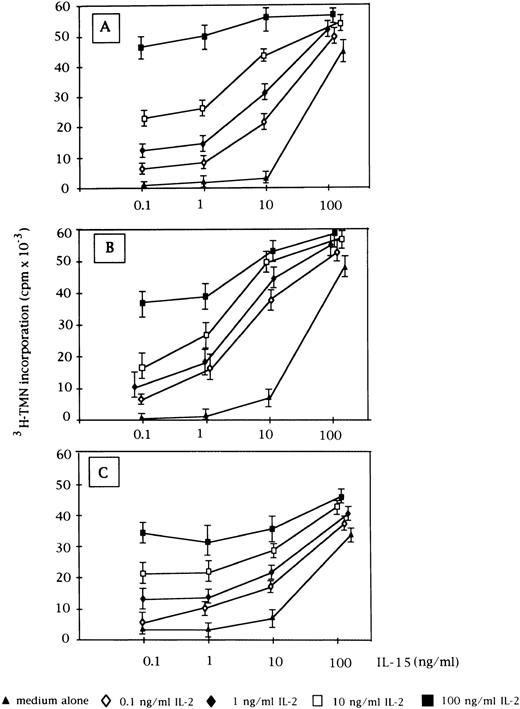
Proliferative activity induced by the combination of IL-2 and IL-15 on CD3+ (A, n = 9), CD3− (B, n = 5), and normal E+-rosetted peripheral blood lymphocytes (C, n = 4). Data are expressed as cpm × 10−3 ± SEM. Fixed concentrations of IL-2 (0.1, 1, 10, and 100 ng/mL) were added to graded doses of IL-15 (0.1, 1, 10, and 100 ng/mL).
Proliferative activity induced by the combination of IL-2 and IL-15 on CD3+ (A, n = 9), CD3− (B, n = 5), and normal E+-rosetted peripheral blood lymphocytes (C, n = 4). Data are expressed as cpm × 10−3 ± SEM. Fixed concentrations of IL-2 (0.1, 1, 10, and 100 ng/mL) were added to graded doses of IL-15 (0.1, 1, 10, and 100 ng/mL).
Cytotoxic activity.Cytotoxic activity was assessed by the lysis of 51Cr-labeled NK-sensitive K-562 cells or NK-resistant Raji in a 4-hour assay, as previously reported.21 Lytic function of GL was analyzed both at resting conditions and following in vitro activation of lymphocytes (106/mL) with IL-2 (10 ng/mL), IL-15 (10 ng/mL), or both cytokines for 72 hours at 37°C in 5% CO2 atmosphere.
Supernatant generation and IL-15 assay.Purified monocytes (1 × 106/mL) from four LDGL (cases no. 2, 4, 12, and 13) and three controls were cultured in 24-well plates (Corning, New York, NY) for 12, 24, and 48 hours at 37°C in 5% CO2 atmosphere. Supernatants were then collected, filtered through a 0.22-μm filter (Millipore, Molsheim, France), and frozen at −80°C until use. Aliquots of supernatants were also concentrated 10-fold using a Centricon Concentrator (Amicon, Beverley, MA). All culture samples and sera were assayed for human IL-15 using two commercially available enzyme-linked immunosorbent assays (Genzyme, Cambridge, MA, and BioSource International, Camarillo, CA) with a sensitivity of 10 and 11 pg/mL, respectively.
RNA extraction, cDNA synthesis, and PCR amplification of IL-2R subunits, CD14, IL-2, IL-15, and IL-15R α-chain mRNA.Total cellular RNA was extracted using the ultraspec-II RNA isolation system (Biotecx Lab, Houston, TX) from cell samples, as previously reported.22 cDNAs were prepared from 2 μg of total cellular RNA by RT at 42°C for 30 minutes in a 20-μL reaction. cDNA was synthesized in the presence of Moloney murine leukemia virus RT (2.5 U), using 2.5 μmol/L oligo-d(T) primer and reaction conditions described by manufacturer (Invitrogen, San Diego, CA). The reaction was stopped by heating the sample to 99°C for 5 minutes.
For the α and β chain of IL-2R, 5 μL of cDNA was amplified in a volume of 50 μL and in the presence of 40 pmol/L of specific primers (Clontech Lab, Palo Alto, CA), which give a band of 398 and 374 bp, respectively. PCR reaction mixture consisted of 1.5 mmol/L MgCl2 , 50 mmol/L KCl, 10 mmol/L TRIS-HCL, 0.2 mmol/L concentrations of each deoxynucleotide triphosphate,and 2.5 U of Taq polymerase (Perkin Elmer, Norwalk, CT). Reaction conditions were 45 seconds melting at 94°C, 45 seconds annealing at 60°C, and 120 seconds extension at 72°C for 30 cycles followed by a final extension for 7 minutes at 72°C in a Cetus/Perkin Elmer thermal cycler. For the γ chain of IL-2R, 1 μL of cDNA was amplified in a total volume of 50 μL in the presence of 25 pmol/L of each oligonucleotide (5′-TATAGGATCCGAAGAGCAAGCGCCATGTTGAAGCC and 3′-AGATTCTGCAGTTTTAGCATCTGTGTGGCC), according to published sequencing data,23 which give an amplification product of 451 bp. The reaction mixture was similar to that for the α and β chain, but contained 2.5 mmol/L MgCl2 . Reaction conditions were 1 minute melting at 94°C, 1 minute annealing at 52°C, and 2 minutes extension at 72°C for 30 cycles followed by a final extension of 7 minutes at 72°C. For control, β-actin gene was amplified from two μL of cDNA in a total volume of 50 μL in the presence of 25 pmol/L of the following oligonucleotides: 5′-GTGGGGCGCCCCAGGCACCA and 3′-CTCCTTAATGTCACG CACGATTTC, which give an amplification product of 540 bp. The reaction mixture was similar to that used for the γ chain. Reaction conditions were 1 minute melting at 94°C, 1 minute annealing at 55°C, and 2 minutes extension at 72°C for 30 cycles followed by a final extension of 7 minutes at 72°C.
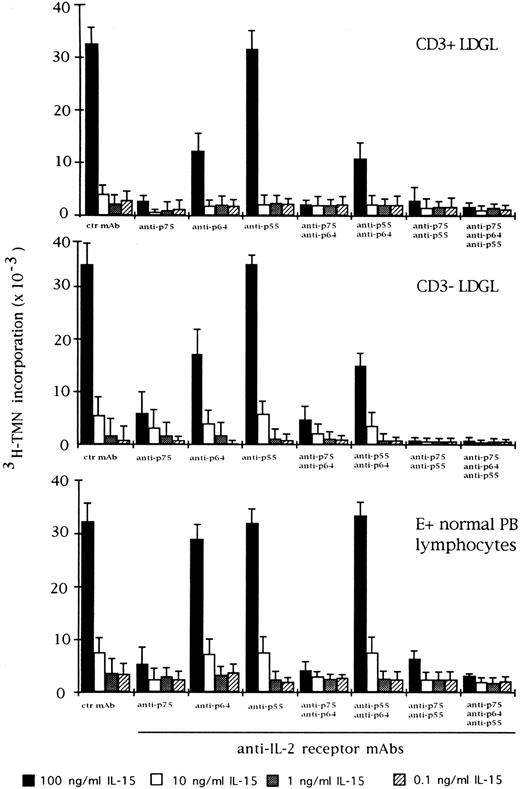
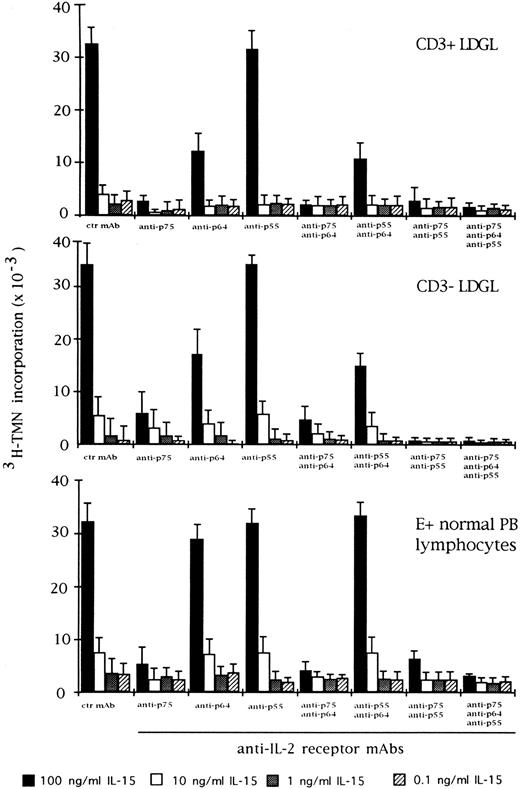
Effect of the blocking of different IL-2R subunits in IL-15–driven proliferation in CD3+ (n = 9), CD3− (n = 5), and normal E+-rosetted peripheral blood lymphocytes (n = 4). Cells were preincubated with BB10 MoAb (anti-p55 IL-2R), TU27 MoAb (anti-p75 IL-2R), and TUGh4 (anti-p64 IL-2R) at the concentration of 10 μg/mL. Data are expressed as cpm × 10−3 ± SEM and compared with the cpm obtained following culture of patients' cells in medium containing control (ctr) isotype IgG MoAbs.
Effect of the blocking of different IL-2R subunits in IL-15–driven proliferation in CD3+ (n = 9), CD3− (n = 5), and normal E+-rosetted peripheral blood lymphocytes (n = 4). Cells were preincubated with BB10 MoAb (anti-p55 IL-2R), TU27 MoAb (anti-p75 IL-2R), and TUGh4 (anti-p64 IL-2R) at the concentration of 10 μg/mL. Data are expressed as cpm × 10−3 ± SEM and compared with the cpm obtained following culture of patients' cells in medium containing control (ctr) isotype IgG MoAbs.
For IL-2 mRNA detection, 3 μL of cDNA was amplified in a total volume of 50 μL in the presence of 25 pmol/L of each oligonucleotide (5′-ACTCACCAGGATGCTCACAT and 3′-AGGTAATCCATCTGTTCAGA), according to published sequencing data,24 which give a band of 266 bp. The reaction mixture consisted of 1.5 mmol/L MgCl2 , 50 mmol/L KCl, 10 mmol/L TRIS-HCL, 0.2 mmol/L concentrations of each deoxynucleotide triphosphate, and 2.5 U of Taq polymerase (Perkin Elmer). Reaction conditions were 1 minute melting at 94°C, 45 seconds annealing at 53°C, and 2 minutes extension at 70°C for 30 cycles followed by a final extension of 7 minutes at 70°C.
For IL-15 mRNA detection, 3 μL of cDNA was amplified in a total volume of 50 μL in the presence of 25 pmol/L of each oligonucleotide (5′-GCCAACTGGGTGAATGTAATAAG and 3′-AATCAATTGCAATCAAGAAGTG), which give an amplification product of 404 bp.25 The reaction mixture was similar to that for IL-2. Reaction conditions were 30 seconds melting at 94°C, 30 seconds annealing at 57°C, and 30 seconds extension at 72°C for 30 cycles followed by a final extension of 7 minutes at 72°C.
For CD14 mRNA detection, 2 μL of cDNA was amplified in a total volume of 50 μL in the presence of 25 pmol/L of each nucleotide (5′-ACTCCCTCAATCTGTCGTTCGCTG and 3′-CTGAAGCCAAGGCAGTTTGAGTCC), according to published sequencing data,26 which give an amplification product of 338 bp. The reaction mixture was similar to that for IL-2. Reaction conditions were 1 minute melting at 94°C, 1 minute annealing at 58°C, and 2 minutes extension at 72°C for 28 to 30 cycles followed by a final extension of 7 minutes at 72°C.
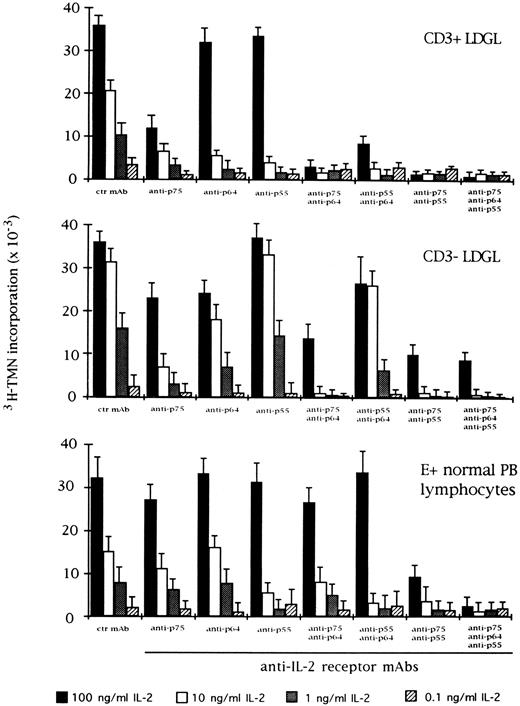
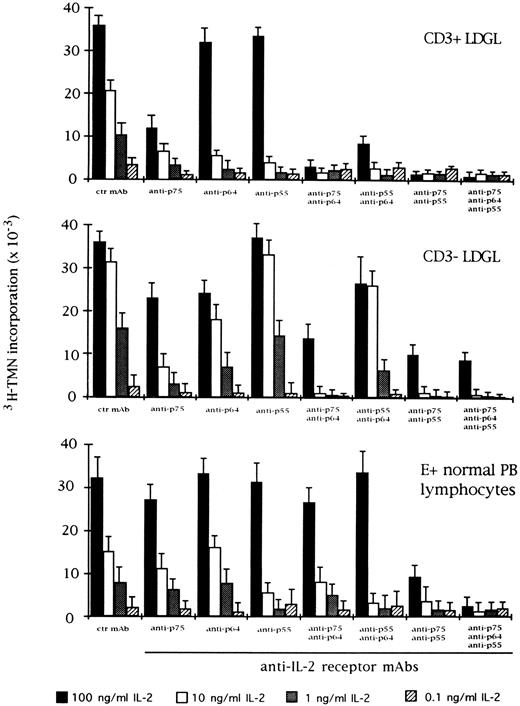
Effect of the blocking of different IL-2R subunits in IL-2–driven proliferation in CD3+ (n = 9), CD3+ (n = 5), and normal E+-rosetted peripheral blood lymphocytes (n = 4). Cells were preincubated with BB10 MoAb (anti-p55 IL-2R), TU27 MoAb (anti-p75 IL-2R), and TUGh4 (anti-p64 IL-2R) at the concentration of 10 μg/mL. Data are expressed as cpm × 10−3 ± SEM and compared with the cpm obtained following culture of patients' cells in medium containing control (ctr) isotype IgG MoAbs.
Effect of the blocking of different IL-2R subunits in IL-2–driven proliferation in CD3+ (n = 9), CD3+ (n = 5), and normal E+-rosetted peripheral blood lymphocytes (n = 4). Cells were preincubated with BB10 MoAb (anti-p55 IL-2R), TU27 MoAb (anti-p75 IL-2R), and TUGh4 (anti-p64 IL-2R) at the concentration of 10 μg/mL. Data are expressed as cpm × 10−3 ± SEM and compared with the cpm obtained following culture of patients' cells in medium containing control (ctr) isotype IgG MoAbs.
For IL-15R α-receptor mRNA detection, 5 μL of cDNA were amplified in a total volume of 25 μL in the presence of 25 pmol/L of each nucleotide (5′-GGCGACGCGGGGCATCAC and 3′-TCGCTGTGGCCCTGTGGATA). According to the published sequence data,27 two amplification products of 531 and 432 bp, which correspond to two mRNA isoforms, including or lacking exon 3, were expected. Amplification conditions were 30 seconds melting at 94°C, 45 seconds annealing at 59°C, and 45 seconds extension at 72°C for 30 cycles followed by a final extension of 7 minutes at 72°C. RNA from PHA-stimulated T lymphoblasts was regarded as a positive control.
Twenty microliters of PCR product was electrophoresed in a 2% agarose gel in Trisborate/edathamil (EDTA) buffer. Gels were stained with ethidium bromide and photographed.
Precaution and controls of PCR analysis.The preparation of all stock solutions and PCR reactions were not performed in the same room in which PCR-amplified products were manipulated. The pipet tips and other equipment used were exclusive for PCR preparation steps. In particular, the pipet tips were plugged and used only once. Aliquots prepared from each stock solution were discarded at the end of a single day's use. Sterile gloves were worn at all times.
Statistical analysis.Data are expressed as the mean ± SEM, and comparisons between values were made using the Cochran-Cox analysis. A value of P less than .05 was accepted as significant.
RESULTS
IL-15 stimulates the proliferation of CD3+ and CD3− GL.Proliferation of resting CD3+ and CD3− GL can be induced by IL-15 in a dose-dependent manner (range, 0.1 to 100 ng/mL) (Fig 1A and B, respectively). The activation was characterized by a rapid increase in proliferation from 10 to 100 ng/mL. For comparison, the proliferation induced by IL-2 is shown for both CD3+ and CD3− GL. A significant difference between the proliferative activities induced by the two cytokines can be documented at the 10-ng/mL concentration. In fact, at this concentration, IL-2 provided a significantly higher proliferation of both CD3+ and CD3− GL. This pattern of activation was consistent with that of normal PBMC, in which a sharp increase in proliferation at a 100-ng/mL IL-15 concentration was reported,28 whereas preactivated peripheral blood T lymphocytes showed a peak of cell proliferation at 10 ng/mL.10
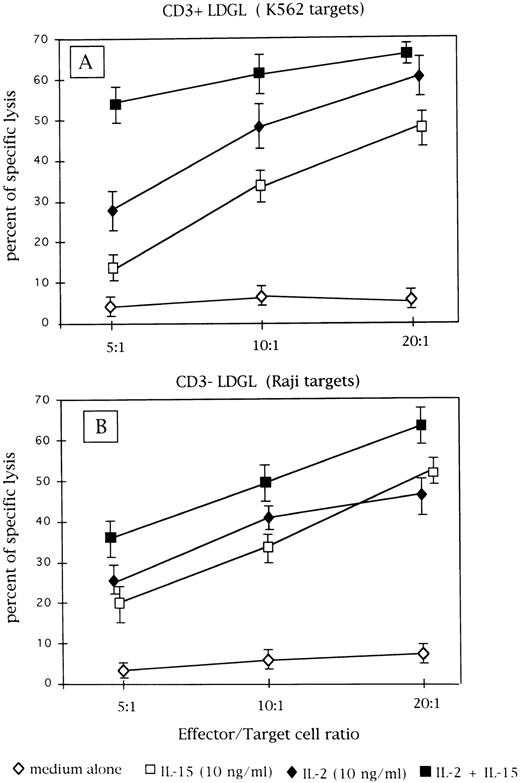
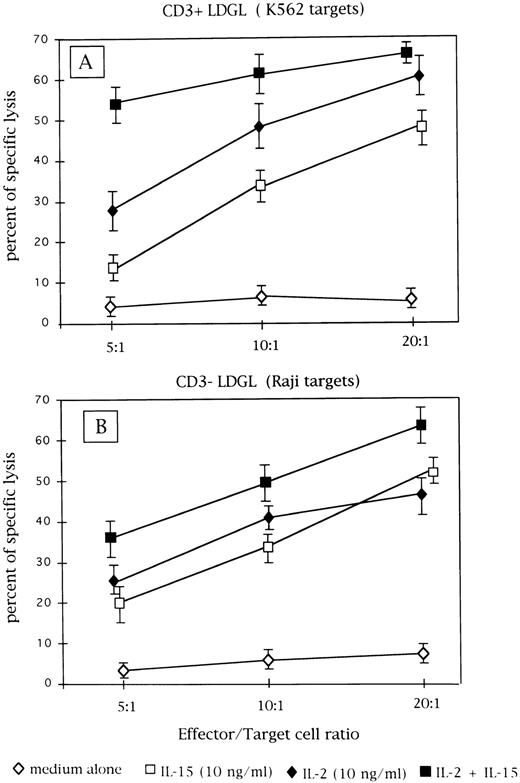
Cytotoxic activity of patients' GL following culture in medium alone or with IL-2 (10 ng/mL) or IL-15 (10 ng/mL) and both cytokines. (A) Results obtained from CD3+ GL (n = 9) against K-562 target cells. (B) Data obtained from CD3− GL (n = 4) against NK-resistant Raji targets.
Cytotoxic activity of patients' GL following culture in medium alone or with IL-2 (10 ng/mL) or IL-15 (10 ng/mL) and both cytokines. (A) Results obtained from CD3+ GL (n = 9) against K-562 target cells. (B) Data obtained from CD3− GL (n = 4) against NK-resistant Raji targets.
When used in combination with IL-2, both cytokines showed an additive effect on cell proliferation, mostly evident at low concentrations (0.1 and 1 ng/mL) up to the plateau level reached by each cytokine at the highest concentration (Fig 2). At that point, the two cytokines were no longer additive. This pattern was demonstrated for both CD3+ and CD3− GL, as well as for normal E+-rosetting cells.
We also examined the effect of anti–IL-2R α, β, and γ MoAbs on IL-15 and IL-2–mediated proliferation in CD3+ and CD3− GL. Antibodies against the β and γ chains of IL-2R were able to inhibit IL-15 activation, particularly in CD3+ GL (Fig 3). On the other hand, preincubation of CD3+ and CD3− GL with anti–IL-2R α chain demonstrated that this receptor was only marginally involved in the proliferation induced by IL-15. The different combination of MoAbs resulted in a variable level of inhibition, with the maximum degree being reached by the contemporary presence of all three MoAbs. This pattern was quite different from that of normal E+-rosetting cells. As shown in Fig 3, the presence of MoAb against the γ chain inhibited the proliferation at a lower degree (10% ± 3% at the highest concentration of IL-15) as compared with the more pronounced inhibition present in LDGL patients.
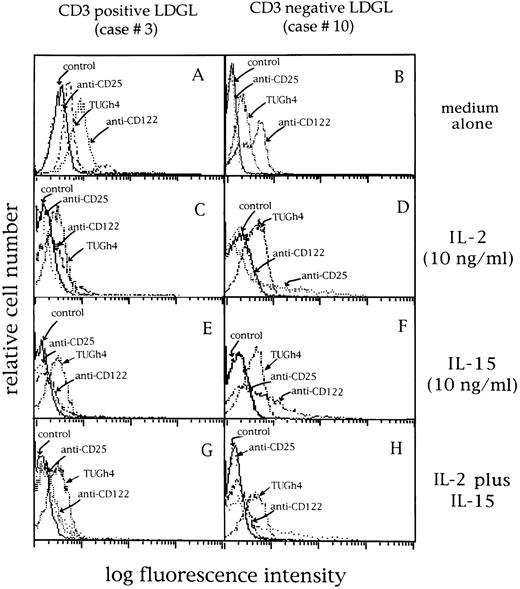
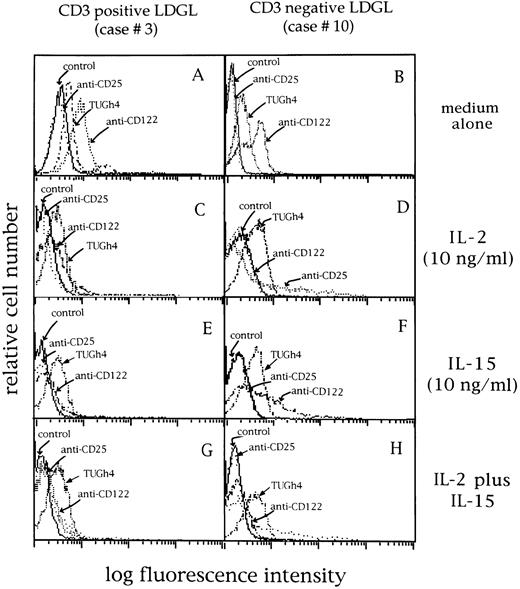
Expression of CD25 (p55 IL-2R, BB10 MoAb), CD122 (p75 IL-2R, Mik-β1 MoAb), and TUGh4 MoAbs (p64 IL-2R) in 2 representative LDGL patients (cases no. 3 and 10). (A and B) Cells cultured in medium alone; the same pattern of expression was shown by freshly isolated patients' GL. (C and D) Cells cultured with IL-2 (10 ng/mL) for 72 hours; (E and F ) cells cultured with IL-15 (10 ng/mL) for 72 hours; (G and H) cells cultured in the presence of both cytokines for 72 hours.
Expression of CD25 (p55 IL-2R, BB10 MoAb), CD122 (p75 IL-2R, Mik-β1 MoAb), and TUGh4 MoAbs (p64 IL-2R) in 2 representative LDGL patients (cases no. 3 and 10). (A and B) Cells cultured in medium alone; the same pattern of expression was shown by freshly isolated patients' GL. (C and D) Cells cultured with IL-2 (10 ng/mL) for 72 hours; (E and F ) cells cultured with IL-15 (10 ng/mL) for 72 hours; (G and H) cells cultured in the presence of both cytokines for 72 hours.
For comparison, the effects of IL-2 and specific anti–IL-2 MoAbs were indicated in the same experimental conditions (Fig 4). As far as the role of the IL-2R γ chain is concerned, preincubation with TUGh4 reduced the proliferative activity of CD3+ and CD3− GL, in particular at an IL-2 concentration less than 10 ng/mL. The combination of anti-β and anti-γ IL-2R MoAbs resulted in an enhancement of inhibition, with the highest levels being reached when the three MoAbs were contemporarily added to the cultures. This pattern was less evident using normal E+-rosetting cells.
Cytotoxic activity induced by IL-2 and IL-15 stimulation.As reported in Fig 5A, CD3+ cases under study showed low levels of cytotoxic activity at resting conditions against K562 target cells; both IL-2 (10 ng/mL) and IL-15 (10 ng/mL) were able to activate cytotoxicity. Since no saturating concentrations of IL-2 and IL-15 were used, the combination of both cytokines induced a further increase in lytic activity. In most instances, CD3− cells showed high values of cytotoxic activity against K562 target cells in the absence of added cytokine; therefore, the NK-resistant Raji cells were used as targets to evaluate cytotoxic activity triggered by IL-2 and IL-15. As reported in Fig 5B, IL-2 and IL-15 stimulated cytotoxic activity against Raji cells with a consistent pattern; the combination of both cytokines showed a cumulative effect.
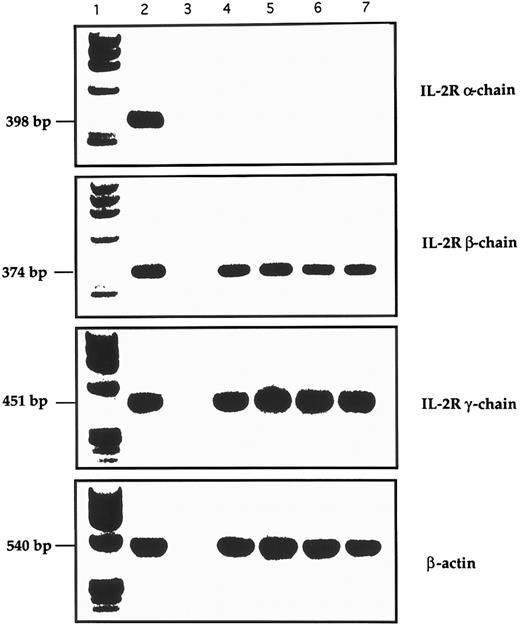
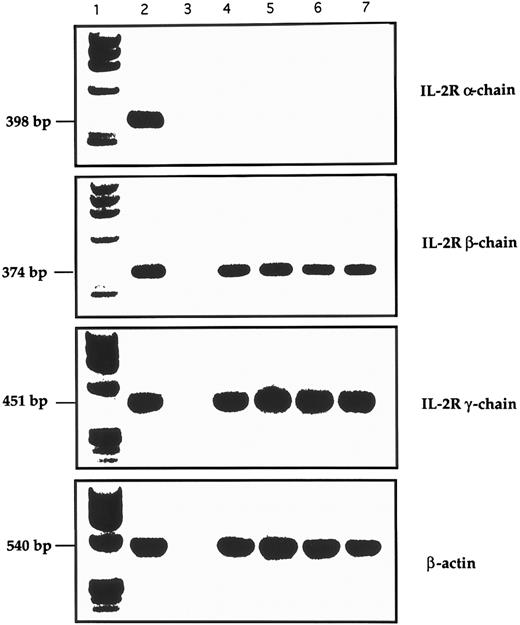
RT-PCR analysis of the expression of IL-2R and control β-actin in GL from 4 LDGL patients. Lane 1, molecular weight marker (φl74 Hae-digested); lane 2, control PBMC stimulated for 12 hours with PHA (5 μg/mL); lane 3, negative control (sample without cDNA); lanes 4 and 5, 2 CD3+ LDGL cases (cases no. 3 and 5 in Table 1); lanes 6 and 7, 2 CD3− LDGL (cases no. 10 and 11 in Table 1).
RT-PCR analysis of the expression of IL-2R and control β-actin in GL from 4 LDGL patients. Lane 1, molecular weight marker (φl74 Hae-digested); lane 2, control PBMC stimulated for 12 hours with PHA (5 μg/mL); lane 3, negative control (sample without cDNA); lanes 4 and 5, 2 CD3+ LDGL cases (cases no. 3 and 5 in Table 1); lanes 6 and 7, 2 CD3− LDGL (cases no. 10 and 11 in Table 1).
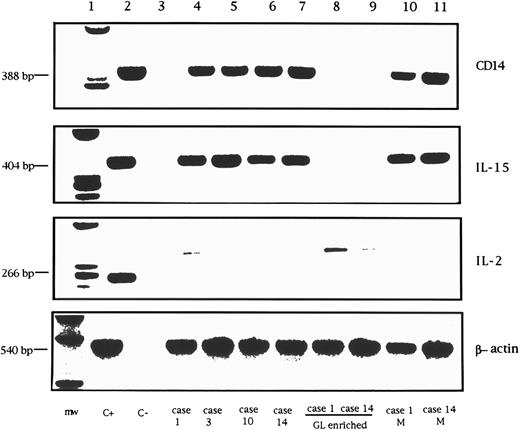
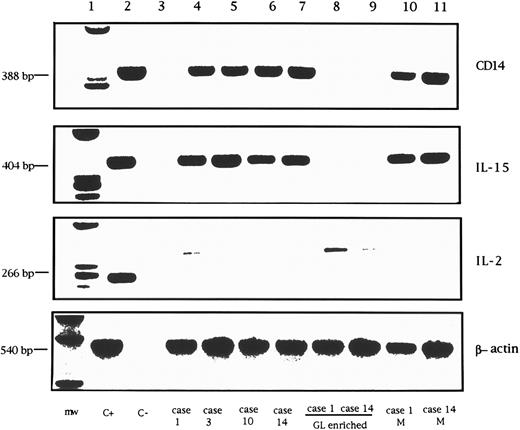
RT-PCR analysis of CD14, IL-15, IL-2, and control β-actin mRNA in GL from 4 LDGL patients. Lane 1, molecular weight marker (φ174 Hae-digested); lane 2, control PBMC stimulated for 12 hours with PHA (5 μg/mL) (positive control); lane 3, negative control (sample without cDNA); lanes 4 and 5, 2 CD3+ LDGL cases (cases no. 1 and 3 in Table 1); lanes 6 and 7, 2 CD3− LDGL (cases no. 10 and 14 in Table 1); lanes 8 and 9, highly enriched GL from cases no. 1 and 14; lanes 10 and 11, enriched peripheral blood monocytes of cases no. 1 and no. 14.
RT-PCR analysis of CD14, IL-15, IL-2, and control β-actin mRNA in GL from 4 LDGL patients. Lane 1, molecular weight marker (φ174 Hae-digested); lane 2, control PBMC stimulated for 12 hours with PHA (5 μg/mL) (positive control); lane 3, negative control (sample without cDNA); lanes 4 and 5, 2 CD3+ LDGL cases (cases no. 1 and 3 in Table 1); lanes 6 and 7, 2 CD3− LDGL (cases no. 10 and 14 in Table 1); lanes 8 and 9, highly enriched GL from cases no. 1 and 14; lanes 10 and 11, enriched peripheral blood monocytes of cases no. 1 and no. 14.
Expression of IL-2R γ chain by CD3+ and CD3− GL.The expression pattern of the IL-2R chains was evaluated by immunofluorescence and RT-PCR. As shown in Table I, patients' CD3+ and CD3− cells expressed the β and γ chains of IL-2R, while α IL-2R was not expressed. Data from two representative patients are shown in Fig 6 (case no. 3, CD3+, Fig 6A; and case no. 10, CD3−, Fig 6B). A consistent pattern of expression for IL-2R resulted from PCR analysis (Fig 7), in which data from two CD3+ and two CD3− patients are shown.
After activation with IL-2 (Fig 6C and D), the expression of p64 chain was not modified (control mean fluorescence intensity (MFI) 68 ± 23 v IL-2 activation MFI 77 ± 34; n = 14), whereas the expression of p75 chain was reduced; p55 chain was induced at very low density (range, 2% to 13%). IL-15 induced a similar pattern (Fig 6E and F ), by down-modulating the expression of p75 chain without affecting the p64 chain expression (MFI, 73 ± 25; n = 14); however, p55 chain expression was unchanged (1% ± 0.4%). The combination of both cytokines (Fig 6G and H) showed a pattern consistent with that of IL-2 alone.
Expression of cytokine mRNA by proliferating CD3+ and CD3− GL.In all patients studied, RT-PCR analysis was unable to demonstrate the expression of IL-15 mRNA in highly purified resting GL. Data from two representative patients are shown in Fig 8 (lanes 8 and 9). At the same time, no signal for IL-2 mRNA was detected in either CD3+ or CD3− GL. On the other hand, a clear-cut IL-15 signal was demonstrated using patients' PBMC, suggesting that cells other than proliferating T or NK cells could account for IL-15 mRNA synthesis. Since monocytes have been reported to represent the source of IL-15 in PBMC,16 the amplification of the monocyte-related CD14 antigen was used to evaluate the presence of contaminating monocytes in GL-enriched populations. We found that the presence of IL-15 mRNA correlated with the presence of CD14+ cells; in fact, in samples devoid of positivity for CD14 mRNA, no evidence of IL-15 mRNA was documented. These data indicate that monocytes likely represent the source of IL-15 mRNA in LDGL patients.
We also evaluated the expression of IL-15 mRNA in patients' cells cultured for more than 6 weeks using IL-2. After this time period, the possibility that contaminating monocytes could still be present in the culture was highly unlikely; however, this was confirmed by the absence of CD14 mRNA expression. Our data confirm that resting and activated CD3+ and CD3− GL do not express detectable mRNA for IL-15.
IL-15 detection in serum and culture supernatants.The presence of IL-15 was evaluated in the sera of patients (n = 14) and no detectable amount of IL-15 could be demonstrated. We also evaluated this cytokine in the supernatants of autologous monocytes and no detectable amount could be demonstrated, even after a 10-fold concentration of samples. No detectable IL-15 could be demonstrated in control cultures (n = 3).
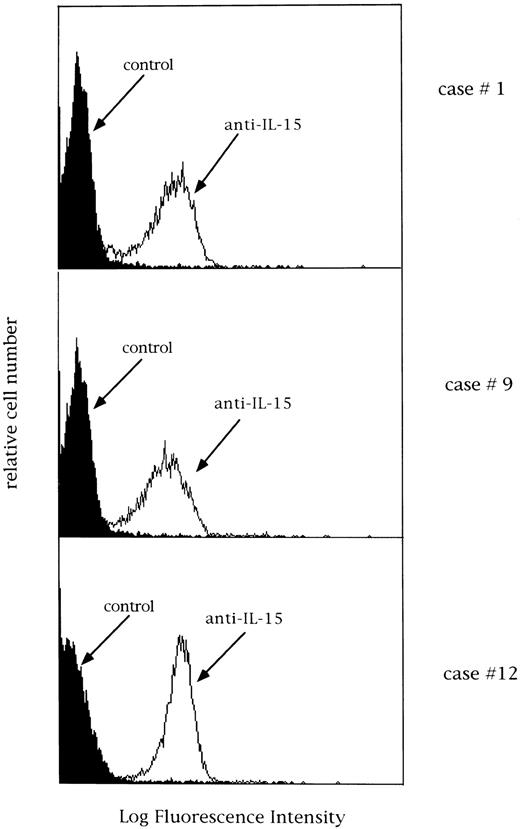
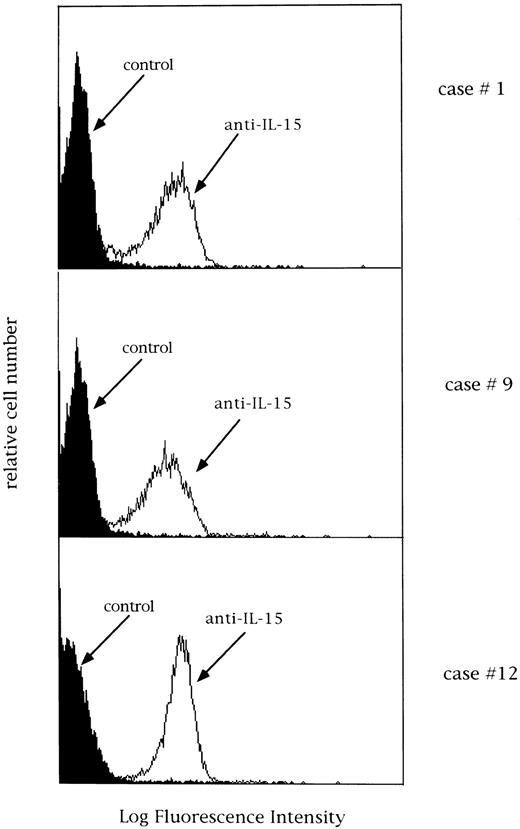
Expression of membrane IL-15 and IL-15R α-chain mRNA.The possibility that a membrane-bound form of IL-15 (mIL-15) could be expressed by patients' GL was explored in selected cases using four different MoAbs against IL-15. Both freshly isolated lymphocytes and cells cultured in medium alone for 24 hours were used. Interestingly, the majority of CD3+ GL (both TCR αβ and γδ) showed the expression of a surface-related IL-15 molecule at high density. We also evaluated the expression of membrane IL-15 in CD3− LDGL cases under the same conditions and demonstrated a definite expression of mIL-15 (Fig 9). In dual-color analysis, the expression of mIL-15 was confined to CD16+ cells.
Expression of IL-15 on 3 different LDGL patients (cases no. 1, 9, and 12 in Table 1). Control histograms (indicated by filled areas) were overlayed on histograms of patients' cells stained with a rabbit anti–15 antibody.
Expression of IL-15 on 3 different LDGL patients (cases no. 1, 9, and 12 in Table 1). Control histograms (indicated by filled areas) were overlayed on histograms of patients' cells stained with a rabbit anti–15 antibody.
Normal peripheral blood T and NK cells were negative for the expression of a mIL-15.
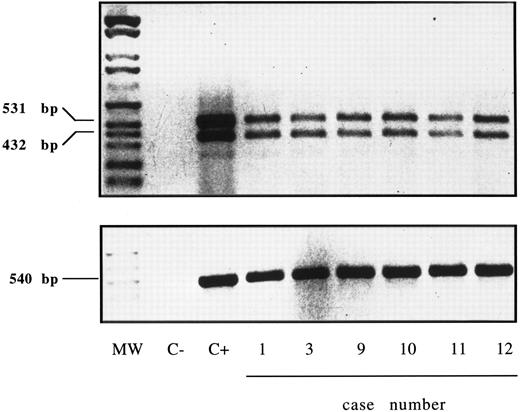
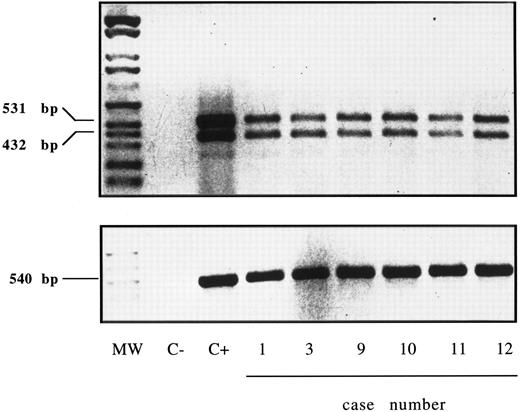
Expression of IL-15R α-chain mRNA was clearly detectable by RT-PCR (Fig 10) in purified GL of six cases (no. 1, 3, 9, 10, 11, and 12), which displayed serum reactivity with anti–IL-15 MoAbs. The presence of two amplification products of 531 and 432 bp is consistent with the existence of two alternatively spliced mRNA isoforms characterized by the presence or absence of exon 3 (± 99 bp).27
RT-PCR analysis of the expression of IL-15R α (upper panel) and control β-actin (lower panel) in highly purified GL from 6 LDGL patients. Lane 1, molecular weight marker (Molecular weight VI–digested); lane 2, negative control (sample without cDNA); lane 3, control PBMC stimulated for 12 hours with PHA (5 μg/mL); lanes 4 to 6, 3 CD3+ LDGL cases (cases no. 1, 3, and 9 in Table 1); lanes 7 to 9, 3 CD3− LDGL (cases no. 10 to 12 in Table 1).
RT-PCR analysis of the expression of IL-15R α (upper panel) and control β-actin (lower panel) in highly purified GL from 6 LDGL patients. Lane 1, molecular weight marker (Molecular weight VI–digested); lane 2, negative control (sample without cDNA); lane 3, control PBMC stimulated for 12 hours with PHA (5 μg/mL); lanes 4 to 6, 3 CD3+ LDGL cases (cases no. 1, 3, and 9 in Table 1); lanes 7 to 9, 3 CD3− LDGL (cases no. 10 to 12 in Table 1).
DISCUSSION
In this study, which was designed to evaluate the functional role of IL-15 in patients with LDGL, we found that (1) IL-15 stimulates the proliferation of GL in a dose-dependent manner and this function is mediated through the p75 and p64 IL-2R chains; (2) IL-15 enhances cytotoxic activity in both CD3+ and CD3− GL; (3) both CD3+ and CD3− GL constitutively express the p64 γ chain of IL-2R; (4) mRNA for IL-15 is detectable in PBMC, but not on highly purified CD3+ and CD3− GL, with monocytes likely representing the source of this cytokine; (5) a membrane-bound IL-15 form is expressed on proliferating CD3+ and CD3− patients' GL surface; and (6) expression of IL-15R α-chain mRNA was clearly detectable by RT-PCR in purified GL. The relevance of these studies must be viewed in relationship to both LDGL and to their counterpart, ie, CD3+ and CD3− normal GL.
The first conclusion of our research is that IL-15 is able to activate CD3+ and CD3− LDGL cells and cooperates with IL-2 in inducing cell proliferation. It is tentatively suggested that in patients with LDGL, IL-15 could contribute to maintaining GL activation at a time in which the production of IL-2 (a specific T-cell product) was downregulated, possibly as a consequence of the putative pathogenetic antigen clearance. The source of IL-15 and the conditions favoring the release of this cytokine in patients with LDGL represent an intriguing issue. The possibility that proliferating GL could themselves produce IL-15, as demonstrated in some lymphoid systems,29 was taken into account. However, the negligibility of IL-15 mRNA in highly purified GL populations (Fig 8), or GL cell lines derived from LDGL patients, argues against this. It will be of interest to verify whether viral agents are able to modulate IL-15 synthesis in these patients. In this regard, recent data point to an important immunoregulatory role for IL-15 in human immunodeficiency virus type-1–infected patients.30 Since LDGL have been suggested to represent chronic proliferations of primed cells against unknown antigens.2,4,31 the analysis of proliferating CD3+ and CD3− GL in LDGL patients could help to clarify some functional properties of their normal counterparts. As for cells belonging to monocytic/macrophage lineage, activated monocytes have recently been demonstrated to release IL-15, which is critical for an optimal production of interferon-γ by NK cells, thus emphasizing the in vivo role of IL-15 during the innate immune response.16 It is tentatively suggested that paracrine production of IL-15 by non-neoplastic monocytes may support the growth of proliferating GL in vivo. However, in vitro experiments of coculture with irradiated monocytes and purified GL were unable to demonstrate a specific role for autologous monocyte in stimulating patients' GL growth (data not shown). In addition, unstimulated monocytes were not prove to release detectable amount of IL-15 in patients with LDGL, although they clearly express IL-15 mRNA. Other studies25,32 have recently reported the difficulty in demonstrating detectable amounts of IL-15 in the supernatants of monocytes that contain IL-15 mRNA, suggesting critical posttranscriptional regulatory events affecting IL-15 expression. The tentative hypothesis is that monocytes in LDGL patients, under specific and still unknown conditions, might rapidly convert IL-15 mRNA from an impeded message into an effectively translatable message. Alternatively, other source(s) of IL-15 production, as recently demonstrated for stromal bone marrow cells,33 should be taken into consideration. These findings also provide additional support to the concept that GL represent a population of cells functionally distinct from cells belonging to the monocytic lineage.34
The presence of the membrane-bound form of this cytokine on the patients' CD3+ and CD3− GL also adds a new piece of information regarding the properties of IL-15, which was not detectable on normal T and NK cells. As in other conditions,35,36 a membrane-bound form of a cytokine can play a crucial role in immune events; in this regard, the observation that the membrane of LDGL cells are equipped with IL-15 could explain why this cytokine could hardly be demonstrated in the cell culture supernatants,25 32 as discussed earlier.
To gain insight into the mechanisms of action and the receptor structures involved in the IL-15–mediated activation in patients with LDGL, we compared the IL-2– with the IL-15–induced activity. Both cytokines were able to stimulate proliferation and enhance cytotoxic activity in CD3+ and CD3− GL. These functions were mostly mediated through the β chain of IL-2R. Interestingly, we and other groups previously reported that CD3+ and CD3− GL in LDGL were constitutively equipped with this IL-2R.7-9 In this study, we expanded these observations by demonstrating the contribution of the recently identified IL-2R γ chain on both IL-15– and IL-2–induced GL activation. Our data indicate that this receptor is constitutively expressed on CD3+ and CD3− GL of LDGL patients. The finding that patients' CD3− GL spontaneously express the IL-2R γ chain suggests that these cells are in an activation state.37 In fact, normal NK cells have been reported to express the IL-2R γ only when activated,37 although some controversy still exists on this issue.19 Finally, although most cases of CD3− LDGL are polyclonal,38 and thus consistent with normal NK cells, it should be kept in mind that a selection of NK subsets was demonstrated in the majority of patients with LDGL regarding the expression of p58 family antigens.21 Studies are in progress to investigate whether IL-15 could favor the expansion of discrete subsets of NK cells in vitro.
IL-15 has been reported to interact with specific receptors, not shared with IL-2R. Using a murine model, Giri et al recently cloned a specific receptor with a high IL-15 binding affinity, indicated as α chain.13 A similar receptor is expressed in human cells27 and contributes to the functional role of IL-15, which is likely to be much more complex than the redundancy of IL-2 function as it results from published studies. In fact, cell lines have been derived that express IL-2R, but are not responsive to IL-15.12 However, the putative α chain of IL-15 seems to be necessary but not sufficient for IL-15 activity.13 By RT-PCR, we clearly demonstrated that purified GL of LDGL patients express the IL-15R α-chain mRNA. Thus, the possibility that IL-15 may bind to GL through IL-15R α and/or IL-2R β/γ remains an attractive hypothesis. However, to the best of our knowledge, no anti–IL-15R α-chain antibodies that are suitable for protein detection on cell surface are available so far, and thus this hypothesis cannot be tested. A better definition of properties and distribution of the α chain will contribute to improve our knowledge regarding the role of IL-15 in the mechanisms that lead to cell proliferation in LDGL.
ACKNOWLEDGMENT
The authors thank Biogen (Cambridge, MA) for supplying recombinant IL-2; Dr A. Troutt from Immunex (Seattle, WA) for providing recombinant IL-15 and anti–IL-15 MoAb M110; Dr K. Sugamura (Senday, Japan) and Dr J. Hamuro (Kawasaki, Japan) for providing TU27 and TUGh4 MoAbs; Dr J. Wijdenes (Besançon, France) for providing BB-10 MoAb; Drs A. Moretta and L. Moretta (Genova, Italy) for providing KD1 MoAb; Dr A. Rosato (Padova, Italy) for providing control rat IgG2a; L. Morosato and A. Rigo for their technical support; Mr Martin Donach for his help in the preparation of the manuscript.
Supported by Centro Nazionale per le Ricerche (CNR), Project on Clinical Application of Oncological Research (Rome), and by Associazione Italiana per la Ricerca sul Cancro (AIRC) (Milan).
Presented in abstract form at the Thirty-Seventh Meeting of the American Society of Hematology, Seattle, WA, December 1995 (Blood 86:322a, suppl 1).
Address reprint requests to G. Semenzato, MD, Università di Padova, Dipartimento di Medicina Clinica e Sperimentale, Via Giustiniani 2, 35128 Padova, Italy.