Abstract
Seven of 112 hemophiliacs infected with human immunodeficiency virus type-1 (HIV-1) before 1986 through contaminated plasma products are currently healthy, with CD4 T-cell counts above 500 cells/μL, and have never received antiretroviral therapy (long-term nonprogressors [LTNPs]). Seven age and sex-matched hemophiliacs infected in the same period but who have progressive HIV disease (progressors) and one additional slow-progressing individual were also studied. One hundred-fold, 20-fold, and 10-fold lower levels of full-length HIV RNA in plasma, peripheral blood mononuclear cells (PBMCs), and proviral DNA in PBMCs, respectively, were found in LTNPs compared with progressors. Plasma and cell-associated HIV RNA and proviral DNA were lower in LTNPs who tested negative for viral isolation from PBMCs or who were positive only after removal of CD8+ cells. No substantial differences were observed in the in vitro production of chemokines including RANTES, MIP-1α, MIP-1β, MCP-1, and interleukin-8 (IL-8) in supernatants of activated PBMCs or CD8-depleted PBMCs of LTNPs, even when HIV isolation was simultaneously accomplished exclusively after removal of CD8+ cells. Low levels of HIV load and replication in peripheral blood are the strongest correlates of nonprogression in this small number of infected hemophiliacs.
HUMAN IMMUNODEFICIENCY virus type-1 (HIV-1) infection leads to a defined pattern of disease progression in most infected individuals. After primary infection, manifesting in some individuals as an acute clinical syndrome, a prolonged period of clinical latency and then clinically overt disease characterized by emergence of opportunistic infections and tumors occurs with an average time of 9 to 10 years.1 During primary infection, several infected CD4+ T cells and a large amount of plasma-associated virus are found in the blood stream.2-5 Then, the amount of circulating virus and viral expression in peripheral blood mononuclear cells (PBMCs) rapidly decline as the humoral and cellular immune responses increase,5-7 marking the beginning of the clinically latent period. The onset of disease coincides with a steeper loss of circulating CD4+ T cells and an increase in cell-associated HIV expression and plasma viremia.1 However, in this scenario, a minority of infected individuals with nonprogressive infection and belonging to all risk categories8,9 have emerged and have been variably defined as long-term nonprogressors (LTNPs), long-term survivors, or long-term asymptomatics. LTNPs are characterized by stable or even increasing CD4+ T-cell counts above 500 cells/μL blood and by strong immune responses, as shown by a high frequency of cytotoxic T lymphocytes against HIV proteins and presence of anti-HIV neutralizing antibodies.10-12 Although substantial agreement exists in describing lower levels of viral replication in LTNPs, remarkable differences in the quantitative levels of plasma-associated HIV RNA have been reported by different investigators, suggesting that LTNPs may indeed represent a heterogeneous group of individuals. A role of human MHC genes in HIV disease progression is highly debated and supported by some studies.13,14 The hypothesis that some LTNPs may possess peculiar immune responses capable of selecting strains of HIV with attenuated pathogenicity or that they had been infected by some of these strains has been suggested in the case of a single LTNP hemophiliac15 and a cohort of a blood donor and six recipients carrying a predominant species of HIV-1 with large deletions in the nef gene.16 These findings reinforce the hypothesis of a crucial role of nef in in vivo viral replication and progression of HIV disease, as previously described in macaques infected with simian immunodeficiency viruses carrying similar deletions.17
Hemophiliacs represent a peculiar population among risk groups, and provide a unique opportunity to investigate progression to AIDS. In fact, they have been exposed to HIV for the limited period during which they were infused with contaminated clotting factor concentrates derived from large pools of plasma. Most hemophiliacs in Italy could have been infected until virus-inactivation methods were introduced in the purification of concentrates in July 1985.18,19 The prevalence of HIV seropositivity in Italian hemophiliacs is 23%,19 additional HIV seroconversions due to transfusions are unlikely to occur, providing a stable cohort. The majority of these patients are evaluated on a frequent and regular basis by the medical staff of a limited number of centers specialized in the treatment of hemophilia. For these reasons, complete information on clinical events, therapeutic interventions, and laboratory parameters are more easily available and study drop-outs are less common than in other risk categories. Furthermore, hemophiliacs are less exposed to sexually transmitted diseases compared with other populations at risk for HIV. Their seroconversion dates are precisely documented because repeated serum samples were collected on a regular schedule before and throughout the period of exposure to HIV infection.
We have examined the virologic state of seven individuals with characteristics of LTNPs selected from a well-characterized cohort of HIV-infected hemophiliacs. These individuals have been matched for sex, age, and duration of infection with seven hemophiliacs with CD4+ T-cell counts less than 200 cells/μL blood and in different stages of disease progression. An additional individual with characteristics of a long-term progressor (LTP), ie, with CD4+ cell counts that had been stable for 12 years but who unequivocally showed signs of disease progression at study entry, was also studied. The infectious capacity of the HIV was measured by viral isolation from plasma, PBMCs, or CD8-depleted PBMCs. Levels of HIV replication in vivo were defined in terms of both plasma and cell-associated viral RNA. The HIV proviral DNA load in PBMCs was quantified in both progressors and LTNPs. Finally, production of chemokines, some of which have been recently shown to suppress HIV replication,20-28 during HIV isolation has been determined in some LTNPs.
SUBJECTS AND METHODS
HIV-Infected Individuals
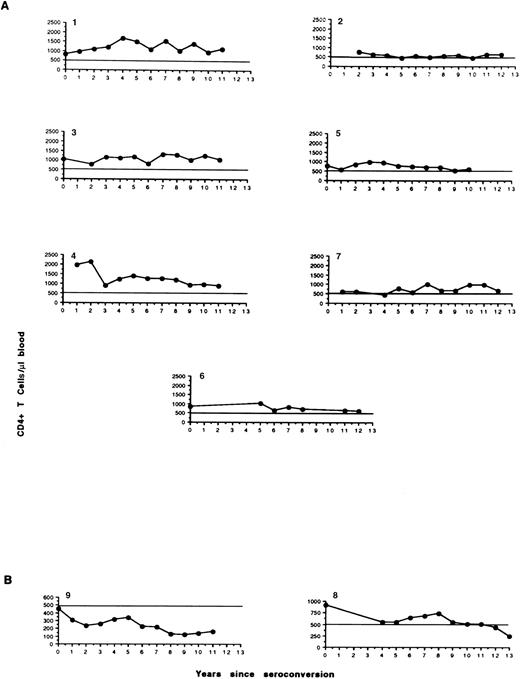
LTNPs.HIV seroconversion was retrospectively analyzed on stored serum samples of 329 hemophiliacs reporting regularly to the A. Bianchi Bonomi Hemophilia and Thrombosis Center in Milan. Seroconversion occurred between 1980 and 1986 in 112 individuals (34%) who were affected by severe hemophilia,29 seven of whom (6.2%) fit the definition of LTNPs according to the following criteria: infection lasting for at least 10 years, absence of HIV-associated symptoms, CD4+ T-cell counts of at least 500 cells/μL blood (Fig 1A), and no antiretroviral therapy. The main characteristics of these LTNPs are shown in Table 1. Four men are affected by severe hemophilia A (patients no. 2, 3, 6, and 7) and two by hemophilia B (no. 1 and 5); one woman (no. 4) is a hemophilia B carrier. The median age of these subjects is 26 years (range, 18 to 46), and their HIV seropositivity was first detected between 1983 and 1985. All LTNPs had chronic hepatitis C virus (HCV) infection. From seroconversion to study entry, the seven patients had always been asymptomatic with undetectable HIV-1 p24 Gag antigen in the plasma as determined by an immunoenzymatic commercial method (Abbott Laboratories, North Chicago, IL). Their CD4+ T-cell counts have been stable for at least 10 years of observation (Fig 1A), and the mean CD4+ and CD8+ lymphocyte counts at study entry were 801 (range, 600 to 1,135) and 1,320 (range, 680 to 1,841) cells per microliter of blood, respectively (Table 1). All LTNPs are in a stable healthy condition with no loss of CD4+ T lymphocytes thus far.
Circulating levels of CD4+ T lymphocytes in hemophilic LTNPs (A). Two peculiar infected hemophiliacs have been included (B). Patient no. 9 showed relatively stable levels of CD4+ T cells over the last 4 years, whereas patient no. 8 has demonstrated a LTNP-like profile up to 11 years since infection, when both the CD4 count and clinical condition have begun to deteriorate (long-term progressor).
Circulating levels of CD4+ T lymphocytes in hemophilic LTNPs (A). Two peculiar infected hemophiliacs have been included (B). Patient no. 9 showed relatively stable levels of CD4+ T cells over the last 4 years, whereas patient no. 8 has demonstrated a LTNP-like profile up to 11 years since infection, when both the CD4 count and clinical condition have begun to deteriorate (long-term progressor).
Hemophiliac LTNPs and Progressors
| Patient No. . | Age (yr)/Sex . | Hemophilia Type . | 1st Anti-HIV+ (yr) . | CD4+ Count (cells/μL)* . | CD8+ Count (cells/μL)* . | CDC Group . | Start Anti-HIV Therapy (yr) . | HLA Type . | |
|---|---|---|---|---|---|---|---|---|---|
| . | . | . | . | . | . | . | . | Class I . | Class II . |
| LTNPs | |||||||||
| 1 | 20/M | B | 1984 | 1,135 | 1,841 | A11, 30; B14, 38 | DR10 | ||
| Cw6; Bw4, 6 | DQ1 | ||||||||
| 2 | 33/M | A | 1983 | 600 | 1,607 | A2, 32; B35, 51 | DR11, 14, 52 | ||
| Cw3, 4; Bw4, 6 | DQ1, 7 | ||||||||
| 3 | 29/M | A | 1984 | 1,047 | 1,806 | A3, 11; B13, 35 | DR1, 7, 53 | ||
| Cw6; Bw4, 6 | DQ1, 2 | ||||||||
| 4 | 18/F | B carrier | 1984 | 905 | 988 | A1; B51, 57 | DR4, 52, 53 | ||
| Cw1, 6; Bw4 | DQ1, 3 | ||||||||
| 5 | 46/M | B | 1985 | 619 | 1,164 | A2; B50, 57 | DR1, 3, 52 | ||
| Cw6; Bw4, 6 | DQ1, 2 | ||||||||
| 6 | 18/M | A | 1985 | 630 | 680 | A3, 33; B13, 14 | DR1, 7, 53 | ||
| Cw6; Bw4, 6 | DQ1, 2 | ||||||||
| 7 | 26/M | A | 1983 | 669 | 1,155 | A3, 11; B44, 51 | DR7 | ||
| Bw4 | DQ2 | ||||||||
| Progressors | |||||||||
| 8 | 42/M | A | 1984 | 445† | 1,886 | A2 | NA | A2, 26; B35, 57 | DR2, 11, 52 |
| Cw4; Bw4, 6 | DQ1, 7 | ||||||||
| 9 | 36/M | A | 1984 | 162 | 1,002 | C2 | 1991 | A25, 28; B18, 35 | DR4, 15, 53 |
| Cw3, 4; Bw4, 6 | DQ1, 3 | ||||||||
| 10 | 27/M | A | 1985 | 105 | 603 | B2 | 1993 | A1, 2; B35, 49 | DR1, 7, 53 |
| Cw4, 7; Bw4, 6 | DQ1, 2 | ||||||||
| 11 | 22/M | A | 1983 | 139 | 1,201 | C2 | 1992 | A2, 26; B35, 51 | DR1, 13, 52 |
| Cw2, 4; Bw4, 6 | DQ5 | ||||||||
| 12 | 49/M | A | 1984 | 122 | 1,314 | A2 | 1992 | A28, 34; B8, 14 | DR1, 3, 52 |
| Cw7; Bw6 | DQ1, 2 | ||||||||
| 13 | 35/M | A | 1983 | 56 | 479 | B2 | 1993 | A24, 29; B7, 44 | DR3, 7, 52, 53 |
| Cw6, 7; Bw4, 6 | DQ1, 2 | ||||||||
| 14 | 33/M | B | 1983 | 69 | 712 | A2 | NA | A24, 32; B62 | DR12, 52 |
| Cw1, 7; Bw4, 6 | DQ1, 7 | ||||||||
| 15 | 35/M | B | 1983 | 31 | 459 | B2 | NA | A3, 33; B14 | DR1, 52 |
| Cw7; Bw6 | DQ1, 3 | ||||||||
| Patient No. . | Age (yr)/Sex . | Hemophilia Type . | 1st Anti-HIV+ (yr) . | CD4+ Count (cells/μL)* . | CD8+ Count (cells/μL)* . | CDC Group . | Start Anti-HIV Therapy (yr) . | HLA Type . | |
|---|---|---|---|---|---|---|---|---|---|
| . | . | . | . | . | . | . | . | Class I . | Class II . |
| LTNPs | |||||||||
| 1 | 20/M | B | 1984 | 1,135 | 1,841 | A11, 30; B14, 38 | DR10 | ||
| Cw6; Bw4, 6 | DQ1 | ||||||||
| 2 | 33/M | A | 1983 | 600 | 1,607 | A2, 32; B35, 51 | DR11, 14, 52 | ||
| Cw3, 4; Bw4, 6 | DQ1, 7 | ||||||||
| 3 | 29/M | A | 1984 | 1,047 | 1,806 | A3, 11; B13, 35 | DR1, 7, 53 | ||
| Cw6; Bw4, 6 | DQ1, 2 | ||||||||
| 4 | 18/F | B carrier | 1984 | 905 | 988 | A1; B51, 57 | DR4, 52, 53 | ||
| Cw1, 6; Bw4 | DQ1, 3 | ||||||||
| 5 | 46/M | B | 1985 | 619 | 1,164 | A2; B50, 57 | DR1, 3, 52 | ||
| Cw6; Bw4, 6 | DQ1, 2 | ||||||||
| 6 | 18/M | A | 1985 | 630 | 680 | A3, 33; B13, 14 | DR1, 7, 53 | ||
| Cw6; Bw4, 6 | DQ1, 2 | ||||||||
| 7 | 26/M | A | 1983 | 669 | 1,155 | A3, 11; B44, 51 | DR7 | ||
| Bw4 | DQ2 | ||||||||
| Progressors | |||||||||
| 8 | 42/M | A | 1984 | 445† | 1,886 | A2 | NA | A2, 26; B35, 57 | DR2, 11, 52 |
| Cw4; Bw4, 6 | DQ1, 7 | ||||||||
| 9 | 36/M | A | 1984 | 162 | 1,002 | C2 | 1991 | A25, 28; B18, 35 | DR4, 15, 53 |
| Cw3, 4; Bw4, 6 | DQ1, 3 | ||||||||
| 10 | 27/M | A | 1985 | 105 | 603 | B2 | 1993 | A1, 2; B35, 49 | DR1, 7, 53 |
| Cw4, 7; Bw4, 6 | DQ1, 2 | ||||||||
| 11 | 22/M | A | 1983 | 139 | 1,201 | C2 | 1992 | A2, 26; B35, 51 | DR1, 13, 52 |
| Cw2, 4; Bw4, 6 | DQ5 | ||||||||
| 12 | 49/M | A | 1984 | 122 | 1,314 | A2 | 1992 | A28, 34; B8, 14 | DR1, 3, 52 |
| Cw7; Bw6 | DQ1, 2 | ||||||||
| 13 | 35/M | A | 1983 | 56 | 479 | B2 | 1993 | A24, 29; B7, 44 | DR3, 7, 52, 53 |
| Cw6, 7; Bw4, 6 | DQ1, 2 | ||||||||
| 14 | 33/M | B | 1983 | 69 | 712 | A2 | NA | A24, 32; B62 | DR12, 52 |
| Cw1, 7; Bw4, 6 | DQ1, 7 | ||||||||
| 15 | 35/M | B | 1983 | 31 | 459 | B2 | NA | A3, 33; B14 | DR1, 52 |
| Cw7; Bw6 | DQ1, 3 | ||||||||
Abbreviation: NA, not applicable.
At study entry.
CD4 cell counts of patient no. 8 decreased to 350/μL shortly after beginning the study.
Progressors.Among patients attending the A. Bianchi Bonomi Center, seven hemophiliacs who were comparable to the LTNPs for age (median, 35 years; range, 22 to 49), sex, and duration of HIV infection but who showed signs of disease progression were studied in parallel. Their CD4+ T-cell counts have been persistently less than 200 cells/μL blood. One additional LTP (patient no. 8) had characteristics of LTNPs up to a few months before the beginning of the study. Unfortunately, he showed a consistent loss of CD4+ T cells in subsequent determinations at 1-month intervals (from 445 cells/μL at study entry to 333 and 350 cells/μL; Fig 1), although with undetectable plasma p24 antigen (Ag) and no clinical signs of HIV-related disease. For these reasons, patient no. 8 was added separately to the control group. Table 1 shows the characteristics of these eight individuals with variably progressing HIV disease. The first HIV seropositivity was dated between 1983 and 1985. All progressors, like LTNPs, are affected by chronic HCV infection. At the beginning of the study, the controls had mean CD4+ T-cell counts of 141 (range, 31 to 445) and CD8+ T-cell counts of 957 (range, 459 to 1,886) cells per microliter of blood, respectively. Values less than 200 CD4+ cells/μL were constantly observed during the past 1 to 5 years (median, 2), with the exception of patient no. 8 (Fig 1B). Plasma levels of p24 Ag have been undetectable in all patients, with the exception of patient no. 11 (maximal peak, 62 pg/mL). The year of seroconversion in both LTNPs and controls was retrospectively determined by Western blot analysis of frozen sera collected between 1980 and 1985. The seroconversion time was determined as the midpoint between the last negative and first positive Western blot determination. Two progressors had developed AIDS (C2 in the classification of the Centers for Disease Control30 ) between 1993 and 1995. Extrapulmonary Mycobacterium tuberculosis infection occurred in patient no. 11, and Pneumocystis carinii pneumonia in patient no. 9. Three of eight progressors (no. 12, 14, and 8) had remained AIDS-free and without signs of other HIV-related disease (CDC-A2) until the beginning of the study. No individuals had ongoing acute diseases when virologic examinations were performed at study entry. Six months after the beginning of the study, full-blown AIDS occurred in two additional patients: progressive multifocal leukoenkephalopathy in no. 14 and P carinii pneumonia in no. 15. Antiretroviral therapy and prophylaxis for P carinii pneumonia had been administered after the decline of CD4+ T cells to less than 300 cells/μL in all but two controls (no. 14 and 15). Both progressors and LTNPs with hemophilia A have been receiving monoclonally purified, high-purity factor VIII concentrate since July 1993, when this product became available in Italy.
PBMCs and CD8-Depleted PBMCs From HIV-Infected Individuals
Peripheral venous blood was collected on both heparin and EDTA. Plasma was prepared by two rounds of centrifugation at 560g at 4°C for 10 minutes, and it was stored in small aliquots at −80°C. PBMCs were prepared by centrifugation on a Ficoll-Hypaque density gradient (Pharmacia Biotech, Uppsala, Sweden). Aliquots of 1 × 106 cells were washed twice with phosphate-buffered saline, and pellets were obtained by centrifugation at 1,083g for 10 minutes and frozen at −80°C for proviral DNA and viral RNA measurements. PBMCs were washed three times with medium and immediately used for viral isolation.
To improve the efficiency of HIV replication, CD8+ cells (mostly T lymphocytes and natural killer cells) were depleted from patients' PBMCs by immunomagnetic beads (Dynatech; Dynal, Oslo, Norway). Freshly isolated PBMCs were resuspended at 20 × 106/mL and incubated with 1 mL immunomagnetic beads conjugated with an anti-CD8 monoclonal antibody at a ratio of one PBMC per seven beads, as recommended by the manufacturer. After 30 minutes of incubation on ice, cell suspensions were depleted of the CD8+ fraction by a magnet. Cells were washed twice with phosphate-buffered saline containing 2% fetal calf serum, and phenotype was analyzed after immunostaining with CD3/CD4, CD3/CD8, CD14, and CD16 monoclonal antibody (Coulter Corp, Hialeah, FL) by an Epics Elite cell sorter (Coulter). CD8-depleted PBMCs contained less than 2% CD8+ cells after a single round of purification (data not shown).
HIV Isolation
Virus was recovered by cocultivation of patients' PBMCs or CD8-depleted PBMCs with phytohemagglutinin 5 μg/mL of (PHA-P; Sigma Chemical Corp, St Louis, MO)-activated PBMCs from uninfected individuals. Two per 106 PBMCs or CD8-depleted PBMCs were cocultivated with a 1:1 mixture of 6 × 106 PHA blasts from two different donors, and resuspended in 12 mL RPMI 1640 (BioWhittaker, Verviers, Belgium) medium containing 10% fetal calf serum (Sigma) and 20 U/mL recombinant interleukin-2 ([IL-2] Boehringer, Mannheim, Germany; complete medium) in T25 flasks (Falcon; Becton Dickinson Labware, Lincoln Park, NJ). Five milliliters of culture supernatants were collected twice per week and stored at −80°C. Fresh complete medium was replaced on a weekly basis, and the cultures were evaluated for a total of 4 weeks. Mg2+-dependent reverse transcriptase (RT) activity was measured in the culture supernatants.31 At peak RT activity, small aliquots of the culture supernatants were stored at −80°C (primary HIV isolates). Supernatants from cocultures that were negative for RT activity were tested for the presence of HIV-1 p24 Ag by a commercially available kit (Coulter). Viral isolation was also attempted from fresh plasma samples collected in the presence of heparin within 30 minutes of isolation. Four milliliters of plasma was added to a 1:1 mixture of 6 × 106 PHA blasts from two different uninfected individuals and the cultures were maintained for 4 weeks, as already described for viral isolation from PBMCs.
Virus Phenotype
The ability of primary HIV isolates to induce syncytia was tested on the human T-lymphotropic virus-1–transformed MT-2 cell line.32 One hundred microliters of viral stocks were used to infect MT-2 cells in 24-well plates (Falcon) containing 2 × 105 cells in 1 mL RPMI 1640 supplemented with 10% fetal calf serum. One hundred microliters of a positive control generated by transfection of the infectious molecular clone pNL4-3 in HeLa cells31 was tested on MT-2 cells in parallel with the patients' isolates. Cultures were monitored daily for syncytia by an inverted light microscope (Olympus Optical Co, Europa, Hamburg, Germany), and RT activity was measured in the culture supernatant to validate whether the absence or presence of syncytia was correlated with a productive infection. Full concordance was observed between the ability of HIV to induce syncytia in MT-2 cells and to give rise to a productive infection as determined by RT or p24 Ag determination (data not shown).
Quantitation of Proviral HIV-1 DNA Molecules
Cellular DNA was obtained from pellets of 106 PBMCs by lysis in a saline solution containing proteinase K and detergents.33 Absolute quantitation of the proviral copies present in 105 PBMCs was performed by competitive polymerase chain reaction (PCR) using the plasmid pSKAN, which contains an 18-bp deleted fragment of the gag region amplified by the primers SK38 and SK39.34 Cell-associated HIV DNA was amplified in the presence of different concentrations of competitor DNA (namely 2,500, 500, 100 and 20 copies) in separate tubes. Amplification and quantitation were performed as previously described.
Quantitation of Genomic Viral RNA in PBMCs and Plasma
Specific HIV-1 transcripts were directly extracted from pellets of 1 to 2 × 106 PBMCs by a previously described single-step method.33 Genomic HIV-1 RNA present in plasma samples was obtained after ultracentrifugation at 4°C for 1 hour at 30,000 rpm (rotor TFT 65.13; Kontron, Milan, Italy), and extraction of pelleted virions was performed as previously described.33 RNA pellets were resuspended in 100 μL diethyl pyrocarbonate–treated water, and the nucleic acid concentration in 105 PBMCs or 100 μL plasma was measured. Quantitation of viral transcripts and cell-free viral genomes was achieved by competitive RT-PCR using the pSKAN-derived RNA competitor.35 Wild-type RNA was mixed with different concentrations of competitor RNA (6,250, 1,250, 250, 50, and, in some cases, 10 copy numbers) in separate tubes. Of note, RNA plasma samples from hemophilic controls had to be diluted to achieve optimal competition. Reverse transcription, PCR amplification, post-PCR procedures, and quantitative analysis of PCR products were performed as described elsewhere.34 35
Cell Activation and Chemokine Determination
PBMCs or CD8-depleted PBMCs from some LTNPs were resuspended in RPMI 1640 plus 10% fetal calf serum, stimulated by PHA plus IL-2 (20/U mL), and seeded at 1 × 106 cells in 24-well plates (Falcon). Culture supernatants were collected 3 days after stimulation and stored at −80°C until tested for the presence of chemokines. Chemokine concentrations were measured either by commercially available enzyme-linked immunosorbent assay (ELISA) kits (R&D Systems Inc, Minneapolis, MN) in the case of RANTES, MIP-1α, and MIP-1β or by a noncommercial ELISA based on anti–MCP-1 or anti–IL-8 monoclonal antibody–coated plastic plates.36 37
RESULTS
HLA typing in LTNPs and controls.HLA class I and II phenotypes were determined by standard serologic typing in LTNP and control HIV-infected hemophiliacs (Table 1). Given the small number of individuals studied, no conclusions can be drawn on the potential role of MHC genes in determining lack of disease progression, as suggested by other studies.13 14
Viremia and cell-associated RNA and proviral DNA.A broad range of viremia levels (between 9,147 and 1,499,843 copies of HIV-1 RNA/mL plasma) characterized individuals with progressing HIV disease. Approximately 300,000 copies/mL were associated with this condition in this cross-sectional analysis (Table 2). In sharp contrast, LTNPs were characterized by levels of HIV-1 viremia consistently less than 10,000 genome molecules/mL plasma as determined by quantitative PCR (between 10 and 8,108 copies), with an approximate mean of 2,500 copies (Table 2). These results are consistent with those reported by Cao et al,10 who indicated a range between less than 630 and 11,549 HIV RNA copies/mL plasma in LTNP homosexuals and intravenous drug users.
Viral Load in LTNP and Progressor Hemophiliacs
| Patient No. . | Plasma* . | PBMC† . | ||
|---|---|---|---|---|
| . | RNA . | RNA . | Proviral DNA . | RNA/DNA . |
| LTNPs | ||||
| 1 | 1,750 | 13 | 11 | 1.2 |
| 2 | 3,840 | 76 | 37 | 2.0 |
| 3 | 8,108 | 144 | 110 | 1.3 |
| 4 | 149 | 2 | 17 | 0.1 |
| 5 | 1,280 | 12 | 28 | 0.4 |
| 6 | 2,120 | 3 | 17 | 0.2 |
| 7 | 10 | 1 | 1 | 1.0 |
| Mean | 2,465 | 36 | 32 | 0.9 |
| Progressors | ||||
| 8 | 100,654 | 50 | 137 | 0.4 |
| 9 | 9,149 | 78 | 18 | 4.3 |
| 10 | 1,499,843 | 1,933 | 397 | 4.9 |
| 11 | 40,537 | 512 | 346 | 1.5 |
| 12 | 18,300 | 292 | 251 | 1.2 |
| 13 | 164,352 | 951 | 753 | 1.3 |
| 14 | 78,870 | 1,978 | 574 | 3.5 |
| 15 | 364,467 | 678 | 300 | 2.3 |
| Mean | 284,521 | 809 | 347 | 2.4 |
| Patient No. . | Plasma* . | PBMC† . | ||
|---|---|---|---|---|
| . | RNA . | RNA . | Proviral DNA . | RNA/DNA . |
| LTNPs | ||||
| 1 | 1,750 | 13 | 11 | 1.2 |
| 2 | 3,840 | 76 | 37 | 2.0 |
| 3 | 8,108 | 144 | 110 | 1.3 |
| 4 | 149 | 2 | 17 | 0.1 |
| 5 | 1,280 | 12 | 28 | 0.4 |
| 6 | 2,120 | 3 | 17 | 0.2 |
| 7 | 10 | 1 | 1 | 1.0 |
| Mean | 2,465 | 36 | 32 | 0.9 |
| Progressors | ||||
| 8 | 100,654 | 50 | 137 | 0.4 |
| 9 | 9,149 | 78 | 18 | 4.3 |
| 10 | 1,499,843 | 1,933 | 397 | 4.9 |
| 11 | 40,537 | 512 | 346 | 1.5 |
| 12 | 18,300 | 292 | 251 | 1.2 |
| 13 | 164,352 | 951 | 753 | 1.3 |
| 14 | 78,870 | 1,978 | 574 | 3.5 |
| 15 | 364,467 | 678 | 300 | 2.3 |
| Mean | 284,521 | 809 | 347 | 2.4 |
Number of copies calculated per mL plasma* or per 105 total PBMCs.†
We also compared levels of HIV expression in the peripheral blood compartment (number of copies of full-length HIV RNA per 105 cells) and viral load (number of copies of proviral DNA per 105 cells) in PBMCs obtained from LTNPs and progressors. As observed in terms of viremia, a clear-cut difference was observed for both of these parameters between the two groups of individuals. Approximately 800 copies of full-length HIV transcripts per 105 cells were found in progressors, in contrast to about 36 copies in equivalent numbers of PBMCs from LTNPs. The proviral DNA load was 10-fold lower in PBMCs of LTNPs than in cells of progressors (Table 2). Of note, the ratio between the level of HIV transcripts and proviral DNA in PBMCs of LTNPs differed substantially from that of progressors. An average ratio of 0.9 (range, 0.1 to 1.3) was found in PBMCs of LTNPs, whereas progressing individuals had a mean ratio of 2.4 (range, 0.4 to 4.9), with seven of eight subjects showing a positive value (Table 2).
HIV isolation and phenotype.The results of our attempts to isolate virus from total PBMCs or CD8-depleted PBMCs of LTNPs and progressors are shown in Table 3. Supernatants from the coculture were first tested for the presence of RT activity, in that this enzyme is 99% particle-associated and its measurement is therefore a reliable index of virion production.31 38 However, this method is substantially less sensitive than determination of p24 Ag by commercially available kits. We therefore performed p24 Ag determinations on culture supernatants that were negative for RT activity. PBMC-associated virus could be successfully recovered from eight of eight individuals with progressive disease but only from two of seven LTNPs (patients no. 2 and 6), as measured by RT activity. Virus isolation from PBMCs was demonstrated by p24 Ag determination in one additional LTNP (no. 3, Table 3). Depletion of CD8+ T cells from PBMCs was performed on cells that did not produce virus during cocultivation with PHA blasts. By this approach, two additional LTNP isolates were obtained, as demonstrated either by RT activity (patient no. 5) or p24 Ag (no. 7) detection (Table 3). Plasma-associated HIV was isolated from six of eight progressors, whereas no isolates were obtained from LTNP plasma (Table 3).
Isolation and Phenotype of Primary HIV Isolates Obtained From Plasma, PBMCs, and CD8-Depleted PBMCs of LTNP and Progressor Hemophiliacs
| Patient No. . | Plasma . | PBMCs . | CD8-Depleted PBMCs . | |||
|---|---|---|---|---|---|---|
| . | RT . | p24 Ag . | RT . | p24 Ag . | RT . | p24 Ag . |
| LTNPs | ||||||
| 1 | — | — | — | — | — | — |
| 2 | — | — | SI | |||
| 3 | — | — | — | NSI | ||
| 4 | — | — | — | — | — | — |
| 5 | — | — | — | — | NSI | |
| 6 | — | — | NSI | |||
| 7 | — | — | — | — | — | NSI |
| Progressors | ||||||
| 8 | NSI | NSI | ||||
| 9 | — | — | NSI | |||
| 10 | — | — | NSI | |||
| 11 | NSI | NSI | ||||
| 12 | NSI | SI | ||||
| 13 | NSI | SI | ||||
| 14 | SI | SI | ||||
| 15 | NSI | SI | ||||
| Patient No. . | Plasma . | PBMCs . | CD8-Depleted PBMCs . | |||
|---|---|---|---|---|---|---|
| . | RT . | p24 Ag . | RT . | p24 Ag . | RT . | p24 Ag . |
| LTNPs | ||||||
| 1 | — | — | — | — | — | — |
| 2 | — | — | SI | |||
| 3 | — | — | — | NSI | ||
| 4 | — | — | — | — | — | — |
| 5 | — | — | — | — | NSI | |
| 6 | — | — | NSI | |||
| 7 | — | — | — | — | — | NSI |
| Progressors | ||||||
| 8 | NSI | NSI | ||||
| 9 | — | — | NSI | |||
| 10 | — | — | NSI | |||
| 11 | NSI | NSI | ||||
| 12 | NSI | SI | ||||
| 13 | NSI | SI | ||||
| 14 | SI | SI | ||||
| 15 | NSI | SI | ||||
Viral isolation was attempted by cocultivation of either plasma or cells from HIV-infected individuals with a mixture of PHA blasts of 2 independent donors. Viral phenotype observed by cultivation of MT-2 cells with HIV isolates.32
HIV isolates from progressors and LTNPs were studied for the capacity to replicate and form syncytia in MT-2 cells.32 Four of five HIV isolates obtained from PBMCs or CD8-depleted PBMCs of LTNPs neither replicated nor induced syncytia, and were therefore classified as NSI (Table 3). Four of eight isolates obtained from progressors' PBMCs were also NSI (Table 3), in agreement with previous studies.39 Five of six isolates from progressors' plasma samples were found to be NSI. Of note, in certain cases (no. 12, 13, and 15), a different and more in vitro aggressive (SI) isolate was obtained from PBMCs compared with plasma (Table 3).
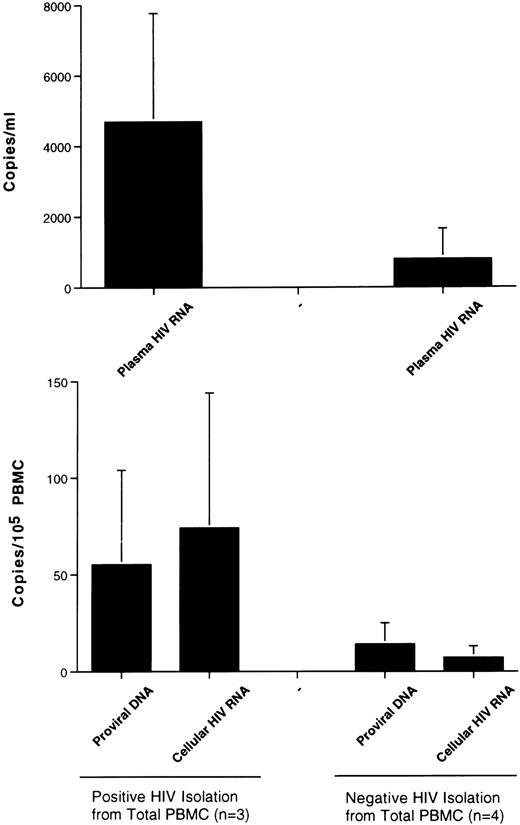
HIV isolation from PBMCs and other virologic parameters in LTNPs.Although the number of LTNPs in our study is small, we have investigated whether the ability to isolate HIV from their total PBMCs (therefore encompassing CD8+ cells with inhibitory capacity for HIV replication)20,40 41 was associated with higher levels of viral load and replication. LTNPs with a positive HIV isolation from unfractionated PBMCs (Table 2) had approximately sixfold higher amounts of HIV RNA in the plasma compared with LTNPs who were either negative for HIV isolation or who were positive only after depletion of CD8+ cells (Fig 2). Eleven-fold and four fold higher levels of expression of cell-associated HIV RNA and proviral DNA load, respectively, were also associated with a positive HIV isolation from unfractionated PBMCs of LTNPs (Fig 2). Due to the small number of LTNPs (three isolation-positive v four isolation-negative), statistical analysis was not performed.
Correlation between HIV replicative parameters and viral isolation from unfractionated PBMCs of LTNP hemophiliacs. Individuals who either were negative for HIV isolation from total PBMCs or became positive only after depletion of CD8+ cells showed lower levels of virus replication in plasma and PBMCs and a smaller number of proviral DNA copies than subjects who were positive for viral isolation from unfractionated PBMCs.
Correlation between HIV replicative parameters and viral isolation from unfractionated PBMCs of LTNP hemophiliacs. Individuals who either were negative for HIV isolation from total PBMCs or became positive only after depletion of CD8+ cells showed lower levels of virus replication in plasma and PBMCs and a smaller number of proviral DNA copies than subjects who were positive for viral isolation from unfractionated PBMCs.
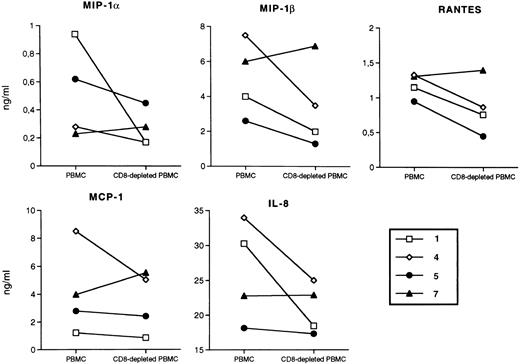
In vitro chemokine production from PBMCs and CD8-depleted PBMCs of LTNPs.Certain chemokines of the C-C family, such as RANTES, MIP-1α, and MIP-1β, have been recently shown to be produced by activated CD8+ T cells20 and to suppress HIV replication both in the PM1 cell line and in primary cells infected in vitro with HIV.20-28 Based on these findings, their role as the predominant mediator of the previously described phenomenon of CD8-dependent nonlytic suppression of HIV replication40,41 has been claimed.20 In addition, increased in vitro secretion of these three chemokines has been observed in CD8-depleted cells of some individuals who had remained HIV-negative despite multiple sexual exposures to the virus, and has been correlated with a relative resistance of the CD4+ T cells to in vitro infection by NSI viruses.21 We therefore have evaluated the concentrations of RANTES, MIP-1α, and MIP-1β, as well as the β-chemokine MCP-1 and the α-chemokine IL-8, after activation of both PBMCs and CD8-depleted PBMCs of some LTNPs by PHA plus IL-2. No substantial differences were observed in the concentrations of these different chemokines in the presence or absence of CD8+ lymphocytes, even in cases (no. 5 and 7) where HIV isolation was simultaneously accomplished exclusively by removal of CD8+ cells (Fig 3). Because our patients, LTNPs and progressors, were all chronically infected with HCV and because whether HCV infection may affect chemokine production is currently unknown, we cannot rule out the possibility that the results shown here have been influenced directly or indirectly by this second pathogen.
Chemokine concentrations in supernatants of PBMCs and CD8-depleted PBMCs of LTNPs stimulated with PHA (5 μg/mL) for 72 hours of culture. Open symbols, HIV isolation positivity from total PBMCs; closed symbols, HIV isolation negativity from unfractionated PBMCs, which either became positive after removal of CD8+ cells (no. 5 and 7), or remained negative (no. 4). No specific patterns of chemokine accumulation in the presence or absence of CD8+ cells were observed. Lower levels of chemokines were observed in cultures of LTNP hemophiliacs after 6 days of culture, whereas comparable levels of chemokines were produced in stimulated PBMC cultures established from uninfected donors.
Chemokine concentrations in supernatants of PBMCs and CD8-depleted PBMCs of LTNPs stimulated with PHA (5 μg/mL) for 72 hours of culture. Open symbols, HIV isolation positivity from total PBMCs; closed symbols, HIV isolation negativity from unfractionated PBMCs, which either became positive after removal of CD8+ cells (no. 5 and 7), or remained negative (no. 4). No specific patterns of chemokine accumulation in the presence or absence of CD8+ cells were observed. Lower levels of chemokines were observed in cultures of LTNP hemophiliacs after 6 days of culture, whereas comparable levels of chemokines were produced in stimulated PBMC cultures established from uninfected donors.
DISCUSSION
In the present study, we describe the virologic characteristics of a homogeneous group of HIV-infected individuals affected by hemophilia and with progressive or nonprogressive (LTNP) infection. LTNPs showed significantly lower viral load and replication as determined by quantitative PCR, with 100-fold, 20-fold, and 10-fold lower levels of HIV plasma viremia, PBMC-associated full-length HIV-1 RNA, and proviral DNA, respectively, compared with progressors. HIV was never rescued from LTNP plasma, whereas six plasma isolates were obtained from controls. HIV was isolated from unfractionated PBMCs of all progressors, and from three of seven LTNPs. The ability to isolate HIV from PBMCs of LTNPs was correlated with higher in vivo levels of HIV load and replication. Two additional isolates were obtained from PBMCs of two LTNPs after depletion of CD8+ cells. Several chemokines, including those recently reported to act as CD8-derived HIV-suppressor factors,20 were detectable in supernatants of cultures established from both PBMCs and CD8-depleted PBMCs of LTNPs. However, no obvious correlations between culture supernatant–associated chemokine levels and the presence or absence of CD8+ cells were observed, even in cases where HIV isolation was accomplished exclusively after removal of this PBMC subset.
LTNPs have been described in all risk categories of HIV-infected individuals. At present, the nature of the relatively apathogenic infection in LTNPs remains elusive. However, a 200-bp deletion of the nef gene has been reported both in a single hemophiliac15 and in a cohort of one donor and six transfusion recipients, all showing nonprogressive disease,16 resembling the attenuated virologic and clinical features of macaques infected with nef-deleted SIV.17 In contrast, no gross deletions of the nef gene have been reported in PBMC-associated proviral DNA obtained from several other LTNPs.42
Some virologic features are consistent among LTNPs belonging to different risk categories evaluated by different investigators in different areas of the world. Substantially lower levels of HIV load in the plasma, likely reflecting virus produced in lymphoid tissue, have been observed in LTNPs compared with progressors. In this regard, a threshold of approximately 10,000 copies of full-length HIV RNA/mL plasma characterizes our population of LTNPs, in close similarity to the population described in a previous study,10 whereas substantially higher levels (70,000 copies/mL) of HIV were indicated in the study by Pantaleo et al.11 Because the quantitative PCR techniques for estimating HIV RNA in plasma and PBMCs in our study and the latter study are identical, it is more likely that these differences truly reflect distinct populations of infected individuals. In this regard, we have selected a homogeneous group of LTNPs in that most of the patients had a history of more than 12 years since seroconversion, in close similarity to the LTNPs described by Cao et al.10 Similar considerations can be made by comparison of the viral load and full-length HIV RNA detected in PBMCs in our study and previous studies.
Despite the fact that HIV RNA was present in the plasma of LTNPs, viral isolation was consistently negative. It is unclear at present whether this finding can be considered an indication that defective HIV predominates in LTNPs, or whether it simply reflects a relatively poor sensitivity of the current methods for isolating HIV in comparison to PCR-based techniques. We could isolate HIV from the plasma of a progressor (patient no. 8) who began to show signs of disease progression at study entry (Fig 1B); of note, this individual had approximately 10 to 50-fold higher levels of HIV RNA than most LTNPs before disease progression. Another patient (no. 9) was characterized by CD4+ T-cell counts of approximately 200/μL over the last 4 years and therefore could be defined a slow progressor (Fig 1B). Quantitation of his plasma-associated HIV showed less than 10,000 copies/mL (Table 2), and HIV isolation from plasma has been repeatedly unsuccessful (Table 3). The virologic state of his PBMCs has confirmed a situation of relative quiescence, with 18 and 78 copies of proviral DNA and transcripts per 105 PBMCs, respectively (Table 2), although HIV could be isolated by cocultivation of unfractionated PBMCs with allogeneic blasts (Table 3). These two cases (no. 8 and 9) highlight the fact that no absolute distinction can be made between LTNPs and individuals with progressing HIV disease based exclusively on virologic parameters, supporting the view that a continuum exists between these two otherwise strikingly diverse outcomes of HIV infection.
In contrast to plasma, infectious HIV could be easily obtained from PBMCs of three of seven LTNPs, whereas it was accomplished in a minority of LTNPs described by Cao et al10 exclusively after CD8 depletion. Viral isolation was accomplished in seven of seven LTNPs studied by Pantaleo et al11 when lymph node mononuclear cells were cocultivated with allogeneic PHA blasts. Unfortunately, no data were reported in this latter study on the ability to isolate HIV from PBMCs of the same individuals. Our observation that progressor patient no. 8 had a still relatively quiescent virologic state at the PBMC level but high levels of plasma-associated culturable virus supports the view that pathogenic events driven by infectious HIV43,44 were occurring in anatomic districts other than the peripheral blood compartment, such as the lymphoid tissue.45 A close virologic follow-up study of patient no. 8 will also investigate whether PBMCs will also present evidence of active viral replication (expression of full-length HIV RNA) and/or spreading (proviral DNA copy number) at subsequent determinations.
Of interest, most PBMCs of LTNPs showed RNA/DNA ratios less than or equal to 1, whereas all but one progressor had positive values indicating an active state of virus expression in the PBMCs (Table 2). In this regard, it has been proposed that a state of “true microbial latency” does not exist in HIV infection,45 and that the finding of cells infected and not actively expressing HIV may simply reflect the kinetics of the virus life cycle in infected CD4+ T lymphocytes.46 In contrast, other studies47 have estimated that the pool of infected cells not expressing HIV at a given time in lymphoid organs, where several factors are in place for favoring viral replication, largely exceeds that of cells actively expressing virus. Our finding that a state of relative quiescent HIV infection characterizes the PBMCs of LTNPs, unlike those of individuals with progressive disease, provides additional evidence of a direct pathogenic link between the extent of virus replication detectable in the peripheral blood compartment and development of AIDS. This concept has found strong supportive evidence in the study by Mellors et al48 showing the power of a single plasma-associated HIV RNA quantitation in predicting the clinical outcome of the patient.
CD8+ cell depletion from the PBMCs of LTNPs has resulted in the rescue of two additional isolates, reinforcing the observation that these T cells often exert a potent suppressive effect on HIV replication.20,40,41 The nature of the suppressive effect has remained elusive for several years, although recent study has begun to shed light on potential mediators. Certain β-chemokines, namely RANTES, MIP-1α, and MIP-1β, are produced by activated CD8+ T cells and cell lines (as well as by many other cell types21,49 ) and suppressed HIV replication both in the PM1 T-cell line and in T-cell blasts of seronegative individuals infected in vitro with different HIV and SIV strains.20 The mechanism of action is explained by the ability of certain chemokine receptors to act as coreceptors together with CD4 for HIV entry.22-28 In addition, IL-16 has been associated with CD8 nonlytic suppression of virus replication in African green monkeys infected with SIVagm .50 In light of these findings, we have determined the presence of chemokines in the supernatants of PBMC cultures and CD8-depleted PBMCs of some LTNPs. All three HIV-suppressive chemokines, as well as MCP-1 (belonging to the same family of β-molecules) and IL-8 (of the α-group of chemokines), were present in detectable amounts in PBMC cultures of LTNPs either directly stimulated by PHA (Fig 3) or cocultivated with allogeneic blasts (not shown). However, no obvious correlations were observed between the presence or absence of CD8+ cells and chemokine concentrations in culture supernatants, and also in those cases where viral isolation was simultaneously accomplished exclusively by removal of CD8+ cells. These results, with the important caveat of noting the small number of individuals who have been evaluated, do not assign a clear-cut role to endogenous chemokines as mediators of CD8-dependent nonlytic suppression of HIV expression during viral isolation. In this regard, PHA-induced production of RANTES, MIP-1α, and MIP-1β, at levels higher and more sustained than those herein reported for LTNPs, was demonstrated in supernatants of CD8-depleted PBMCs of two exposed uninfected individuals who were resistant to in vitro infection by NSI HIVs.21
The ability to isolate HIV from LTNP PBMCs was inversely correlated with the three virologic parameters investigated in the present study. LTNPs who tested negative for HIV isolation from their PBMCs or who became positive only after CD8+ cell depletion had substantially lower levels of HIV load and replication (cell-associated transcripts) in PBMCs than LTNPs who tested positive (Fig 2). These results reinforce the hypothesis that LTNPs represent a heterogeneous group of individuals, and that virologic markers may represent the best predictors of rapid or slow disease evolution.48 Molecular analysis of the HIV quasi-species present in vivo and obtained in vitro from this group of hemophiliacs is in progress, with particular focus on the potential presence of variants of the HIV-1 nef allele.15-17
In conclusion, our study has demonstrated that hemophiliacs with nonprogressive HIV disease are characterized by substantially lower levels of HIV load and replication in the peripheral blood compartment than a matched group of hemophiliacs with progressing HIV disease. Although our study confirms the view that the extent of HIV replication in vivo likely is “the major determinant of pathogenicity,”1,43-46,48,51 the immunologic and virologic components involved in either pathogenic or relatively apathogenic HIV infection remain uncovered.
ACKNOWLEDGMENT
We thank Sergio Bernasconi for the quantitation of MCP-1 and IL-8 in culture supernatants.
Supported by grants from the 1995 AIDS Project of the Istituto Superiore di Sanita', Rome, Italy.
Address reprint requests to Elisa Vicenzi, Laboratori P2/P3, DIBIT, Via Olgettina n. 58, 20132, Milan, Italy.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal