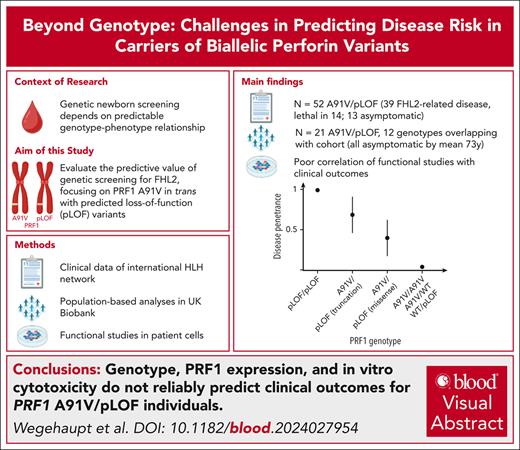
Key Points
Variable penetrance of A91V/pLOF PRF1 genotypes complicates disease prediction.
Functional assays do not reliably predict clinical outcome.
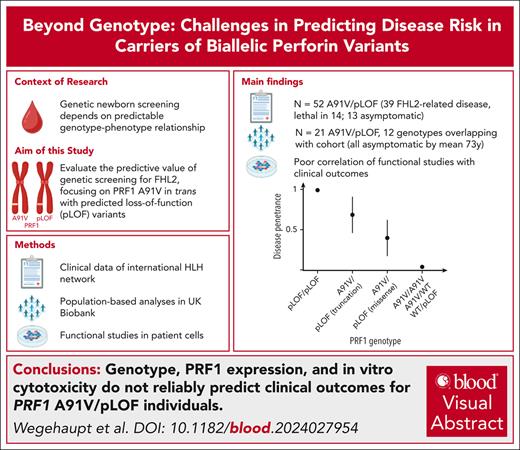
Visual Abstract
Genetic screening for severe congenital immunohematological diseases offers potential for early intervention, particularly through preemptive allogeneic hematopoietic stem cell transplantation (HSCT). However, the clinical value of such screening depends on precise prognostic predictions based on genotype-phenotype correlations and/or functional confirmation. We investigated familial hemophagocytic lymphohistiocytosis type 2 (FHL2), caused by PRF1 variants. Specifically, we evaluated the clinical significance of the frequent PRF1 A91V variant, if present in trans with a predicted loss-of-function (pLOF) PRF1 variant, defined as “disease mutation” listed in the Human Gene Mutation Database. We combined clinical and functional data from our hemophagocytic lymphohistiocytosis (HLH)–network registry with UK Biobank data to evaluate disease penetrance and clinical outcomes. Among 52 individuals with A91V/pLOF genotype in the registry, 39 (72%) showed FHL2-related manifestations with mean onset at 20 years. Four patients had recurrent disease, 15 received transplantation, and 14 died. Among 14 individuals with A91V/pLOF genotype identified by family screening (mean age, 29 years), however, only 1 was symptomatic. Moreover, among 21 A91V/pLOF carriers identified in 200 000 UK Biobank participants, 12 with genotypes identical to symptomatic registry patients, none had developed HLH by age 73 years. Premature stop pLOF alleles appeared more penetrant than missense variants, but functional data including perforin expression or cytotoxicity failed to predict disease manifestation. Our combined registry and population-based approach reveals significant variability in disease penetrance and severity among PRF1 A91V/pLOF carriers, with no clear association between genotype, functional data, and clinical outcomes. This complexity illustrates the challenges of genetic screening and highlights the need for careful clinical decision-making regarding preemptive HSCT in asymptomatic carriers.
Introduction
Monogenic diseases can present with significant phenotypic heterogeneity even among individuals with variants in the same gene. The effect of a specific variant on protein function plays a crucial role in determining disease manifestation and severity. Hypomorphic gene variants, which result in reduced but not completely absent protein function, can lead to phenotypes ranging from full disease manifestation to subclinical symptoms or no apparent disease.1 Individual prediction of disease risk becomes particularly challenging in these situations. This is even more relevant in genetic diseases, in which external triggers contribute to disease manifestations. However, accurate risk assessment is crucial for providing informed genetic counseling, in particular if identifying at-risk infants through newborn screening (NBS) programs for early interventions.2
Perforin deficiency, caused by pathogenic variants in the PRF1 gene, is a recessively inherited inborn error of lymphocyte cytotoxicity (familial hemophagocytic lymphohistiocytosis type 2 [FHL2]).3 Null variants in PRF1 lead to absent cytotoxicity and predispose to systemic hemophagocytic lymphohistiocytosis (HLH) that invariably manifests in infancy or early childhood.4 The risk of HLH recurrence is very high, such that allogeneic hematopoietic stem cell transplantation (HSCT) is required as curative therapy.5 Hypomorphic PRF1 variants allowing residual cytotoxicity can lead to later onset HLH in adolescence or adulthood.6-8 These patients can also present with chronic inflammatory phenotypes including demyelinating inflammatory central nervous system disease, chronic cytopenia, recurrent fever and splenomegaly, aplastic anemia, or lymphoma.9-12
The common PRF1 A91V polymorphism (c.272 C>T; NM_001083116.3) is a hypomorphic variant affecting perforin folding and stability13,14 and is carried by ∼8% of the European population.15,16 A91V in heterozygous or homozygous forms impairs cytolytic activity up to 50%,14,17 but population-based studies have revealed that it does not increase the risk for HLH, inflammatory disease, cancer, or premature death.15,18 Uncertainty remains whether this is also true for cases in which PRF1 A91V pairs in trans with variants that are known to cause HLH in homozygosity or compound heterozygosity. Throughout this paper, we designate these known disease-causing alleles as “predicted loss-of-function” (pLOF) PRF1 variants. Individual patients with HLH with A91V/pLOF have been described,7,19-31 but in these cases the contribution of the genetic defect to HLH is difficult to prove and the risk of HLH recurrence has not been systematically investigated, rendering recommendations on HSCT difficult. Because infants with perforin deficiency are usually born healthy and transplantation results are more favorable in asymptomatic patients,32,33 FHL2 is an important candidate for NBS. However, because of the high frequency of the A91V variant, a relevant prevalence of A91V/pLOF compound heterozygous combinations is expected in the population, calling for a better understanding of disease risk in such individuals.
Here, using 3 approaches, we analyzed whether PRF1 A91V/pLOF predisposes to HLH or other FHL2-related diseases: (1) analysis of a clinical cohort of PRF1 A91V/pLOF cases identified through our international HLH network and literature research; (2) analysis of individuals with PRF1 A91V/pLOF identified through the population-based UK Biobank (UKB); and (3) analysis of the functional consequences of PRF1 A91V/pLOF in primary patient cells. Our data show that in this particular allele constellation, neither genetics nor functional assays can fully predict the risk to develop FHL2-related disease manifestations. The overall low risk justifies regular monitoring but argues against preemptive HSCT in all cases. However, because of the high lethality, this should be considered for symptomatic patients.
Methods
Study patients
This study was approved by the local ethics committee of the Medical Center University of Freiburg (approval number 22-1170_1). Informed consent for HLH registries or clinical studies was present for all individuals recruited to the clinical cohort. The UKB study was approved by the North West multicenter research ethics committee. This study has been conducted using the UKB Resource under application number 103789.
Recruitment of individuals with A91V/pLOF genotype via physician network and literature search
To recruit individuals with A91V/pLOF genotype, we approached HLH referral centers, promoted the study through the Histiocyte Society and contacted colleagues at conferences. Patients were included if they carried the A91V variant (c.272 C>T) in trans with a pLOF PRF1 variant, defined as “disease mutation” listed in the Human Gene Mutation Database (HGMD).34 Patients with A91V/pLOF+pLOF (>1 variant in trans to A91V) and A91V/A91V+pLOF (homozygous A91V with 1 additional monoallelic PRF1 variant) genotypes were also eligible. The following information was collected: sex, age at last clinical information, reason for genetic testing, perforin variant other than A91V, disease characteristics (episodes of HLH or other FHL2-related disease manifestations, age at onset, and disease triggers), drug therapy, HSCT, and outcome including cause of death. Six patients evaluated for suspected primary HLH had an A91V/pLOF genotype but no further clinical information was available. These patients were not included in the cohort but were included in studies evaluating the relationship between genotype and immune function.
In addition, we performed a PubMed literature search for A91V/pLOF cases (search terms “perforin AND a91v” or “compound∗ AND a91v”), including a search among references in identified articles.
Population-based identification of individuals with A91V/pLOF genotype in UKB
To identify participants with a A91V/pLOF genotype at the population level, we made use of the UKB database that comprises nearly 500 000 adult volunteer participants recruited between 2006 and 2010, when aged 40 to 69 years, for whom both their disease history (in form of International Classification of Diseases 10th Revision [ICD-10]–coded diagnoses) as well as their whole-genome sequencing data were available.35 Among these, biallelic genome data (“phasing” of alleles) was available for 200 000 participants (supplemental Table 1, available on the Blood website). A second analysis additionally included variants listed as “possible disease mutations” in HGMD.
We established a list of 72 ICD-10 codes describing possibly FHL2-related phenotypes (supplemental Table 2), including HLH, neuroinflammatory disease,9,10,20,36 lymphoma,30,31,36 chronic cytopenia,20 aplastic anemia,12 and liver disease.20,37 For 1 part of the analysis, UKB participants with any of these ICD-10 codes were defined as the “target group” (N = 3840), whereas all remaining UKB participants (N = 195 076) served as the control group. Additional statistical and bioinformatics methods are described in the supplemental Methods.
Functional studies
Intracellular perforin staining was performed on peripheral blood mononuclear cells, as previously described.38 All assays were performed with the identical protocol in a single, experienced, diagnostic laboratory that is an acknowledged reference center for HLH diagnosis and analyzes >100 patient samples per year. Each patient sample was analyzed in parallel to a travel control sample. The mean fluorescence intensity of perforin in control samples is continuously monitored and, if outside the reference values, the staining of the patient sample is not considered valid. Reference values for the mean fluorescence intensity of perforin expression have been established in 120 healthy control samples across different age groups. Natural killer (NK)–cell cytotoxicity assays were performed in the same laboratory with peripheral blood mononuclear cells isolated from fresh blood using a 51Cr release on K562 target cells according to a standard protocol described in the supplemental Methods. NK-cell activity was normalized to the fraction of NK cells in the sample. We only included assays that were performed in HLH-free episodes. Normal values were obtained in 2 different time periods and were based on 40 healthy donors. For cytotoxic T lymphocytes (CTL) cytotoxicity, long-term T-cell blasts were used. T-cell blast generation and CTL cytotoxicity assays were performed according to a standard protocol described in the supplemental Methods.
Results
Clinical cohort of individuals with A91V/pLOF genotype
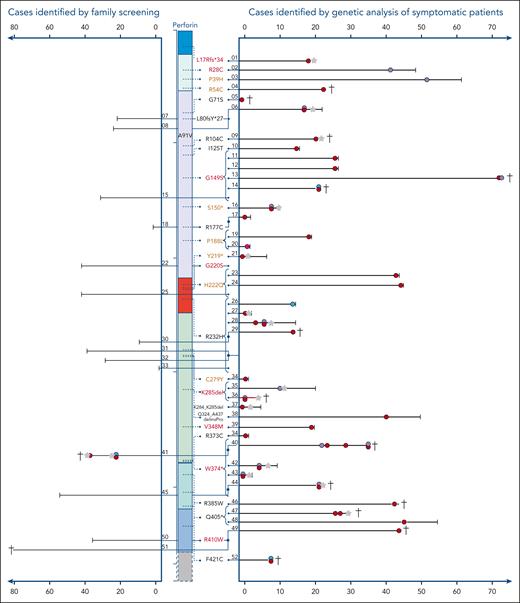
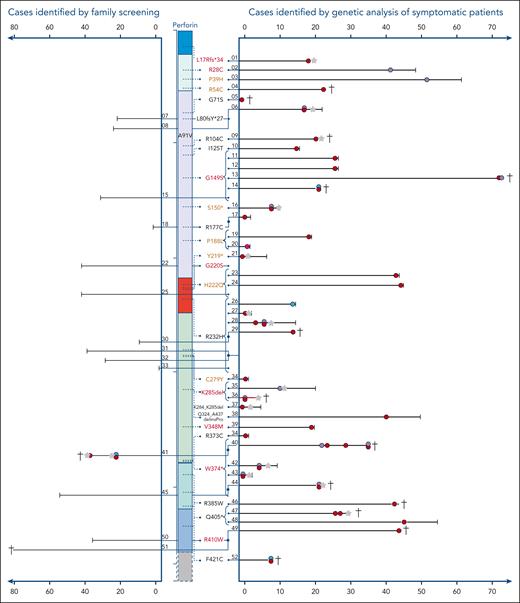
Overall, 39 patients with HLH or other likely FHL2-related phenotypes and 13 asymptomatic individuals were reported by 13 centers from 7 countries. Nineteen patients had been published previously7,19-31 and 33 are reported for the first time (Table 1). Of 39 symptomatic patients, 34 (87%) had developed systemic HLH at some point in their clinical history (Figure 1), 22 as their only FHL2-related clinical manifestation. Ten patients (including all 4 with A91V/W374∗ and both with the A91V/K285del genotype) showed additional neuroinflammatory symptoms. Two patients presented with T-cell lymphoma–associated systemic HLH. Isolated inflammatory phenotypes not fulfilling HLH criteria were reported for 2 patients with neuroinflammation,9 1 patient with recurrent febrile episodes, and 1 patient with T-cell lymphoma.
Cohort of A91V/pLOF cases. Schematic illustration of the perforin gene with its functional domains. On the right side, the variants are indicated that were observed in trans with the A91V variant. Variants in red cause HLH in homozygosity (pLOF/pLOF), “pLOF” in brown cause HLH in compound heterozygosity (pLOF1/pLOF2), pLOF marked in black have not been linked to FHL2-associated phenotypes in the literature. Each line represents the longitudinal course of 1 individual with A91V/pLOF genotype in years of life. The patient identification number is indicated. Right part: cases identified by genetic analysis of symptomatic patients. Left part: cases identified by family screening. Red circle: episode of systemic HLH or HLH-like inflammation. Purple circle: neuroinflammatory symptoms, either isolated or as part of systemic HLH. Green circle: lymphoma. Pink circle: recurrent febrile episodes not meeting HLH criteria. Gray star: HSCT. Black vertical line: age at last clinical information. †patient deceased. Connected lines with a junction point symbolize individuals belonging to the same family. Brackets pointing to variants summarize groups of patients carrying this A91V/pLOF genotype.
Cohort of A91V/pLOF cases. Schematic illustration of the perforin gene with its functional domains. On the right side, the variants are indicated that were observed in trans with the A91V variant. Variants in red cause HLH in homozygosity (pLOF/pLOF), “pLOF” in brown cause HLH in compound heterozygosity (pLOF1/pLOF2), pLOF marked in black have not been linked to FHL2-associated phenotypes in the literature. Each line represents the longitudinal course of 1 individual with A91V/pLOF genotype in years of life. The patient identification number is indicated. Right part: cases identified by genetic analysis of symptomatic patients. Left part: cases identified by family screening. Red circle: episode of systemic HLH or HLH-like inflammation. Purple circle: neuroinflammatory symptoms, either isolated or as part of systemic HLH. Green circle: lymphoma. Pink circle: recurrent febrile episodes not meeting HLH criteria. Gray star: HSCT. Black vertical line: age at last clinical information. †patient deceased. Connected lines with a junction point symbolize individuals belonging to the same family. Brackets pointing to variants summarize groups of patients carrying this A91V/pLOF genotype.
Potential disease triggers in patients with systemic HLH included infection with Epstein-Barr virus (EBV; n = 8), cytomegalovirus, mycoplasma, enterovirus (n = 1 each), and lymphoma (n = 3). No disease trigger was detected in 16 cases (8 children, and 8 adults), information was missing in the remaining 5 patients. Three patients had asymptomatic seroconversion to EBV before disease manifestation. Disease onset was variable with a mean onset of 20 years (range, neonatal to 72 years; 19 in childhood and 20 in adulthood). Of 39, 7 patients had >1 temporally independent FHL2-related inflammatory episode before definitive therapy, last clinical information, or death, emphasizing the genetic predisposition to disease manifestations. Therapy mostly included HLH-94 or HLH2004 protocols,39,40 single patients received alemtuzumab, JAK inhibition, anakinra, tocilizumab, and/or sirolimus. Central nervous system HLH was treated with intrathecal steroids and methotrexate. Fifteen patients (38%) underwent allogeneic HSCT; 8 had a favorable outcome, 5 died from transplant-related complications, and in 2 patients HSCT-outcome was not reported. Notably, 9 of 24 (37%) patients that did not receive transplantation died of progressive HLH activity, multiorgan failure, infections, or lymphoma. Thus, from this case collection, A91V/pLOF emerges as a genotype associated with severe FHL2-related manifestations, a high risk of recurrence, and high lethality.
We also sought to report A91V/pLOF individuals identified by family screening of symptomatic index patients with FHL2 (pLOF1/pLOF2). Surprisingly, of 14 A91V/pLOF cases identified (5 parents, 7 siblings, and 2 extended family members), 13 (3 children and 10 adults) had not developed any FHL2-related symptoms within the observation period (mean, 29 years [range, 1-81]). One A91V/pLOF sibling had a history of T-cell lymphoma and inflammatory episodes fulfilling HLH criteria. For 3 individuals, asymptomatic EBV seroconversion was documented.
In the A91V/pLOF cohort, 27 different PRF1 variants were reported. Fourteen of these variants were detected in homozygosity or compound heterozygosity in patients with FHL2-related disease. Among the remaining 13 variants, 5 were classified as disease mutations and 1 as possible disease mutation in the HGMD, respectively, whereas 7 were not listed in the HGMD. The latter were included because, although not yet formally listed in the HGMD, they fulfilled the definition of disease mutation used in this database. Five PRF1 variants in symptomatic patients with A91V/pLOF were detected in >1 independent family (G149S, R232H, K285del, W374∗, and Q405∗). For 5 PRF1 variants we found both symptomatic and asymptomatic individuals with A91V/pLOF genotype across different families (G149S, P188L, H222Q, R232H, and W374∗).
A91V/pLOF population genetics
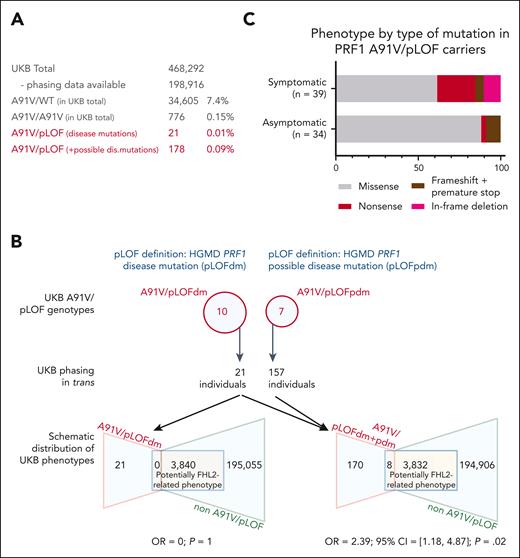
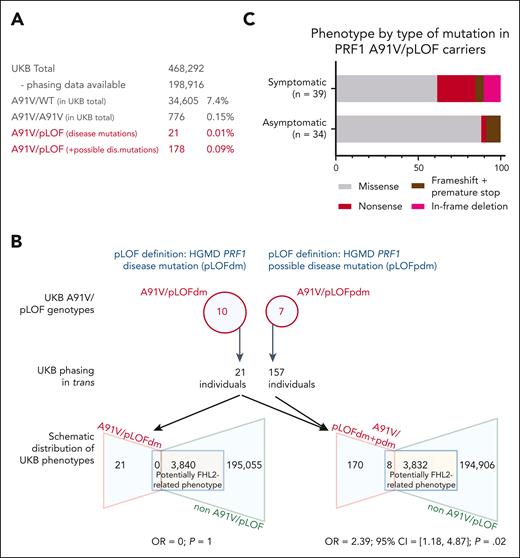
The clinical cohort of individuals with A91V/pLOF genotype was inevitably subject to recruitment bias, because most cases were collected from HLH referral centers and genetically tested because of FHL2-related phenotypes. To obtain more unbiased data, we decided to study individuals with an A91V/pLOF genotype of advanced age at the population level. In UKB, the PRF1 A91V variant was detected in 7.4% of all participants (Figure 2A), in line with previous reports.15,16 Among the 34 605 heterozygous A91V carriers, 2 had a history of HLH (ICD code D76.1 or D76.2). Homozygosity for A91V was observed in 0.15% participants (n = 776), none of whom had experienced HLH. The allele frequencies of heterozygous (A91V/wild-type [WT]) and homozygous A91V (A91V/A91V) carriers were not enriched in UKB participants with HLH (n = 15) or other possibly FHL2-related phenotypes (n = 9385). Five A91V/pLOF genotypes overlapped between the clinical cohort and genetically “phased” individuals in the UKB (pLOF HGMD disease mutations: R28C, R177C, and G220S; pLOF not in HGMD: G71S and L80fsY∗27). They were observed in 8 individuals of the clinical cohort, 3 with systemic HLH, 1 with HLH-related disorders, and 4 asymptomatic family members. In contrast, none of the 13 individuals in the UKB carrying these genotypes (11 with a HGMD disease mutation, and 2 with a pLOF not listed in HGMD) had any evidence of the potentially FHL2-related phenotypes at a mean age of 74 years in their records (range, 61-84).
A91V/pLOF in the UKB. (A) Prevalence of various PRF1 A91V allele constellations in the UKB. Prevalence of A91V/WT and A91V/A91V is calculated for the UKB total. Prevalence of A91V/pLOF (with in trans phasing) is calculated in phased UKB individuals only. (B) Upper part shows PRF1 variants listed in the HGMD as disease mutations (pLOFdm) and possible disease mutations (pLOFpdm) and present in coinheritance with A91V in the UKB. Lower part shows distribution of potentially FHL2-related phenotypes in A91V/pLOF and non-A91V/pLOF individuals in the UKB for A91V/pLOFdm only (lower left) or disease and possible disease mutations combined (lower right, A91V/pLOFdm+pdm). Indicated is the OR and 95% CI for an individual with A91V/pLOF genotype to be located in the target group of potentially FHL2-related phenotypes. (C) Phenotype by type of PRF1 pLOF mutation in trans to A91V. Percentage distribution of 39 symptomatic A91V/pLOF carriers and 34 asymptomatic A91V/pLOF carriers (13 in clinical cohort and 21 in the UKB with HGMD disease mutation in trans to A91V). 95% CI, 95% confidence interval; OR, odds ratio.
A91V/pLOF in the UKB. (A) Prevalence of various PRF1 A91V allele constellations in the UKB. Prevalence of A91V/WT and A91V/A91V is calculated for the UKB total. Prevalence of A91V/pLOF (with in trans phasing) is calculated in phased UKB individuals only. (B) Upper part shows PRF1 variants listed in the HGMD as disease mutations (pLOFdm) and possible disease mutations (pLOFpdm) and present in coinheritance with A91V in the UKB. Lower part shows distribution of potentially FHL2-related phenotypes in A91V/pLOF and non-A91V/pLOF individuals in the UKB for A91V/pLOFdm only (lower left) or disease and possible disease mutations combined (lower right, A91V/pLOFdm+pdm). Indicated is the OR and 95% CI for an individual with A91V/pLOF genotype to be located in the target group of potentially FHL2-related phenotypes. (C) Phenotype by type of PRF1 pLOF mutation in trans to A91V. Percentage distribution of 39 symptomatic A91V/pLOF carriers and 34 asymptomatic A91V/pLOF carriers (13 in clinical cohort and 21 in the UKB with HGMD disease mutation in trans to A91V). 95% CI, 95% confidence interval; OR, odds ratio.
We then extended our analysis to additional pLOF perforin alleles that were not present in our clinical cohort. Using HGMD, we identified 203 PRF1 variants defined as disease mutations. Among these, we found 79 in the UKB; 10 of which were coinherited in trans with A91V in additional 10 of 198 916 participants (A91V/pLOF; Figure 2B). Unexpectedly, none of them had a record of systemic HLH or other FHL2-related phenotypes at a mean age of 73 years (range, 58-84).
To be even more inclusive, we then also considered possible disease PRF1 mutations as defined by the HGMD (n = 43; Figure 2B). A total of 91 UKB participants were homozygous for such variants (R4H, n = 61; R4C, n = 2; A211V, n = 1; A437V, n = 4; N252S, n = 22; R357Q, n = 1); only 2, both homozygous for R4H, had a possibly FHL2-related phenotype (multiple sclerosis and hepatomegaly, respectively). Indeed, several of these variants have been characterized as probably neutral polymorphisms (R4H; A211V; N252S) or variants of unclear significance (A437V).7,14,41,42 Nevertheless, when including these possible disease variants in our pLOF definition, we found an enrichment of A91V/pLOF carriers among phased individuals with possibly FHL2-related phenotypes including splenomegaly/cytopenia (n = 3), neuroinflammation (n = 1), or lymphoma (n = 4) compared with all remaining UKB participants (8/3832 vs 170/194 883; odds ratio, 2.39; 95% confidence interval, 1.18-4.87; P = .02). Notably, none of the 17 UKB genotypes was exclusively present in symptomatic individuals, but all of these were also present in the control group (supplemental Table 3). Taken together, the cumulated prevalence of A91V/pLOF in the UKB ranged between 0.01% (HGMD disease mutations only) and 0.09% (HGMD disease and possible disease mutations combined; Figure 2A).
Although these population-based data indicated that the A91V/pLOF genotypes as a group conferred only a low risk for FHL2-related manifestations, the possibility remained that the risk varied between different pLOF alleles. We therefore compared the type of pLOF variants in trans to A91V in all symptomatic vs asymptomatic individuals pooled from the cohort and from the UKB. Indeed, we found that the more deleterious variants (nonsense, frameshift leading to premature stop, deletions) were enriched in the symptomatic (15/39, 38%) compared with the asymptomatic mutation carriers (4/34, 12%). This suggests that the nature of the pLOF variant pairing with A91V matters and more deleterious variants are associated with increased risk (Figure 2C).
Functional studies in primary A91V/pLOF lymphocytes
We then analyzed whether protein expression or function could be of prognostic value.
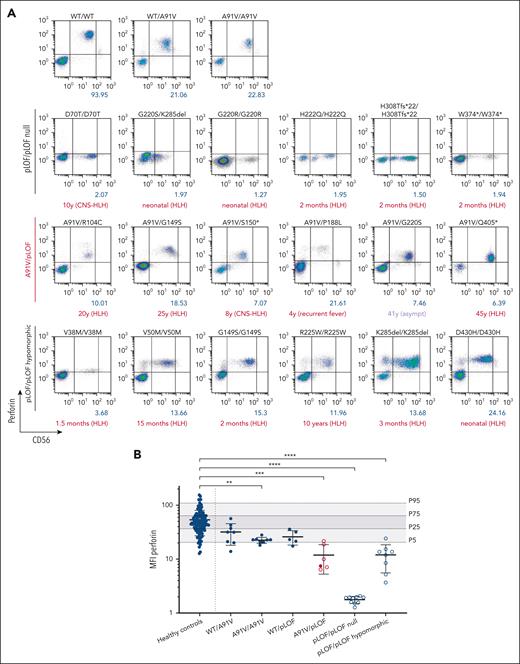
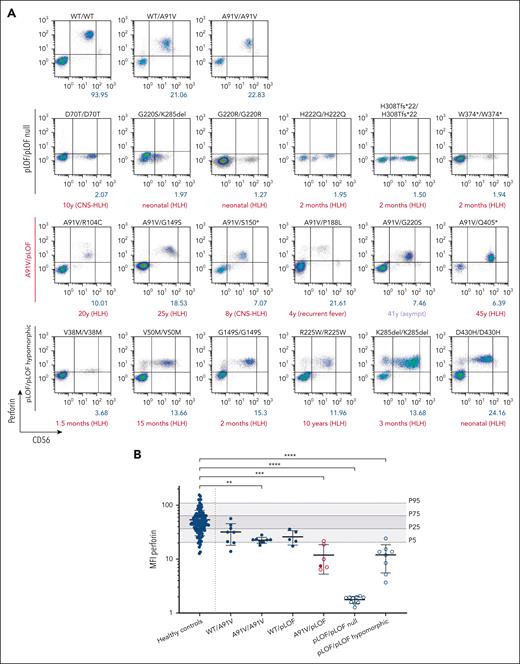
We analyzed samples of 5 patients with HLH (patient 9 [P9], A91V/R104C; P11, A91V/G149S; P16, A91V/S150∗; P17, A91V/R177C; and P48, A91V/Q405∗), 1 patient with recurrent febrile episodes not fulfilling HLH criteria (P20, A91V/P188L), and 2 asymptomatic individuals (P18, A91V/R177C; P22, A91V/G220S) in a single reference laboratory under standardized conditions. Except for the genotypes of the 2 asymptomatic cases (in which we found corresponding genotypes in 2 and 1 asymptomatic UKB participants, respectively), these genotypes were exclusively present in our clinical cohort. Perforin expression was detectable but significantly reduced to a similar extent in NK cells of 5 symptomatic and 1 asymptomatic individuals with different A91V/pLOF genotypes (Figure 3A). Notably, perforin expression in the low penetrance A91V/pLOF constellation was lower than in NK cells from individuals with A91V/WT, A91V/A91V, or WT/pLOF genotypes but similar to levels of NK cells from patients with 8 biallelic hypomorphic disease-causing PRF1 variants (Figure 3B). These data suggest that the level of perforin expression is of limited value in predicting disease risk in individuals with the A91V/pLOF genotype.
Intracellular perforin expression in patient-derived A91V/pLOF CD3−CD56+ NK cells. (A) Flow cytometry dot plots of perforin expression levels in CD3−CD56+ NK cells with different PRF1 genotypes. First row: PRF1 WT, A91V/WT, and A91V/A91V carriers. Second row: homozygous PRF1 “null” variants (pLOF/pLOF null). Third row: PRF1 A91V/pLOF genotypes. Fourth row: homozygous PRF1 hypomorphic variants (pLOF/pLOF hypomorphic). Perforin mean fluorescence intensity (MFI) is shown in blue, age of onset of clinical manifestations and phenotype is shown in red below the panels. (B) Perforin MFI for different PRF1 genotypes. Percentile 5, 25, 75, and 95 of perforin expression in 120 nonsequenced healthy controls are indicated by gray areas. Filled circles, asymptomatic individuals; empty circles, symptomatic individuals. Ordinary 1-way analysis of variance of perforin MFI between healthy controls and PRF1 variant carriers (∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001).
Intracellular perforin expression in patient-derived A91V/pLOF CD3−CD56+ NK cells. (A) Flow cytometry dot plots of perforin expression levels in CD3−CD56+ NK cells with different PRF1 genotypes. First row: PRF1 WT, A91V/WT, and A91V/A91V carriers. Second row: homozygous PRF1 “null” variants (pLOF/pLOF null). Third row: PRF1 A91V/pLOF genotypes. Fourth row: homozygous PRF1 hypomorphic variants (pLOF/pLOF hypomorphic). Perforin mean fluorescence intensity (MFI) is shown in blue, age of onset of clinical manifestations and phenotype is shown in red below the panels. (B) Perforin MFI for different PRF1 genotypes. Percentile 5, 25, 75, and 95 of perforin expression in 120 nonsequenced healthy controls are indicated by gray areas. Filled circles, asymptomatic individuals; empty circles, symptomatic individuals. Ordinary 1-way analysis of variance of perforin MFI between healthy controls and PRF1 variant carriers (∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001).
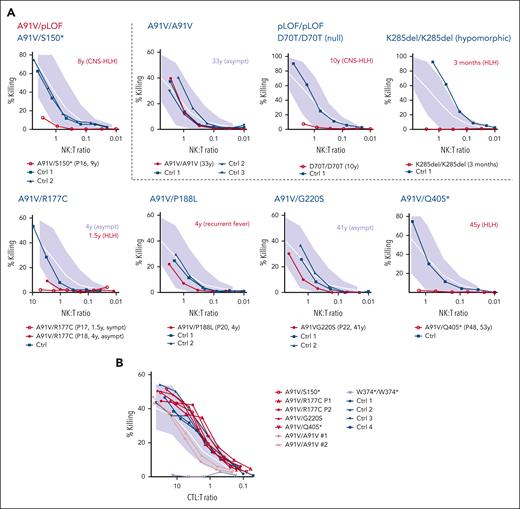
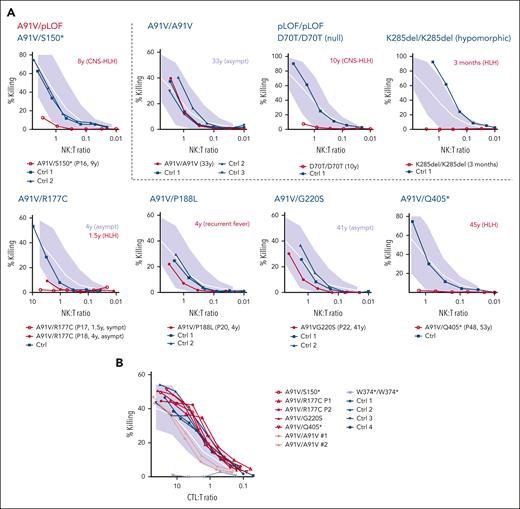
NK-cell cytotoxicity may be impaired during acute HLH episodes and recover after convalescence,43 which we also observed in 3 different A91V homozygous individuals (supplemental Figure 1). We therefore only evaluated NK-cell cytotoxicity assays that were performed in the absence of acute HLH. Cytotoxicity was reduced in 4 cases with 3 genotypes (P16, A91V/S150∗; P17 and P18, A91V/S177C; and P48, A91V/Q405∗). Although P16, P17, and P48 had experienced HLH, P18 was asymptomatic. Cytotoxicity was normal in 2 further individuals (P20, A91V/P188L; and P22, A91V/G220S), who were asymptomatic (Figure 4A). In contrast to cytotoxicity performed in fresh NK cells, cytotoxicity of in vitro–stimulated A91V/pLOF CD8 T cells was normal in individuals with all 4 investigated A91V/pLOF genotypes, including 3 symptomatic patients (Figure 4B). These results indicate that cytotoxicity assays cannot unambiguously predict the risk for FHL2-associated disease.
Cytotoxicity of A91V/pLOF cytotoxic lymphocytes. (A) NK-cell cytotoxicity assessed by 51Cr release of peripheral blood mononuclear cells (PBMC) incubated with K562 target cells for 4 hours. The NK-to-target ratio(NK:T) was calculated after flow cytometric quantification of NK cells among PBMC. Spontaneous release was <15%. Experiments were performed in HLH-free episodes of patients with the indicated genotypes in comparison to nonsequenced healthy controls (Ctrl; dark blue). Patient identification numbers are shown in parentheses below the panels. Age of onset of clinical manifestations and phenotype is shown in red next to the panels. (B) CTL cytotoxicity assessed by 51Cr release of phytohemagglutinin/interleukin-2–stimulated T-cell blasts incubated with anti-CD3–labeled L1210 target cells for 4 hours. Cells from patients with the indicated genotypes were analyzed in comparison with nonsequenced healthy ctrl (dark blue). Empty circles: patients with FHL2-related phenotype. Filled circles: asympt individuals. Gray area indicates mean (white line) and 2 standard deviations of cytotoxicity obtained with T cells from healthy ctrl. Asympt, asymptomatic; CTL:T, cytotoxic T cell-to-target ratio; sympt, symptomatic.
Cytotoxicity of A91V/pLOF cytotoxic lymphocytes. (A) NK-cell cytotoxicity assessed by 51Cr release of peripheral blood mononuclear cells (PBMC) incubated with K562 target cells for 4 hours. The NK-to-target ratio(NK:T) was calculated after flow cytometric quantification of NK cells among PBMC. Spontaneous release was <15%. Experiments were performed in HLH-free episodes of patients with the indicated genotypes in comparison to nonsequenced healthy controls (Ctrl; dark blue). Patient identification numbers are shown in parentheses below the panels. Age of onset of clinical manifestations and phenotype is shown in red next to the panels. (B) CTL cytotoxicity assessed by 51Cr release of phytohemagglutinin/interleukin-2–stimulated T-cell blasts incubated with anti-CD3–labeled L1210 target cells for 4 hours. Cells from patients with the indicated genotypes were analyzed in comparison with nonsequenced healthy ctrl (dark blue). Empty circles: patients with FHL2-related phenotype. Filled circles: asympt individuals. Gray area indicates mean (white line) and 2 standard deviations of cytotoxicity obtained with T cells from healthy ctrl. Asympt, asymptomatic; CTL:T, cytotoxic T cell-to-target ratio; sympt, symptomatic.
Discussion
Genetic perforin deficiency is the most severe known risk factor for HLH. Biallelic loss-of-function variants in the PRF1 gene lead to early onset of this life-threatening disease. Most affected children are born healthy and their identification before onset of inflammatory symptoms, for example, by a prospective genetic NBS, promises opportunities for preemptive curative HSCT. However, this requires clear guidelines on prognosis and treatment of individuals carrying any combination of biallelic perforin variants, including those allowing residual perforin function. Previous studies showed that the polymorphism A91V (7.4% in the UKB) does not cause FHL2-related symptoms when present in homozygosity or in trans with a WT allele.15 This study focused on genotypes in which A91V pairs with a definite or probable loss-of-function perforin allele. We found such genotypes to be present in 0.09% of the general population. In countries with ∼700 000 annual births such as Germany, the United Kingdom, or France, NBS would identify 630 infants with such a genotype. What would be the appropriate management strategy for these babies?
Our study revealed a striking contrast between the high disease burden observed in symptomatic clinical cohorts and the apparent absence of disease in population-based samples. These differences could not just be explained by the specific PRF1 pLOF genotypes. Among 39 symptomatic A91V/pLOF patients, 26 specific genotypes were identified, 9 of which were also carried by 24 asymptomatic individuals in the cohort or UKB participants. The advanced age of these asymptomatic individuals suggests that their lack of disease cannot be explained by limited observation periods. Furthermore, 5 genotypes were observed in both asymptomatic and symptomatic individuals within the same family.
The lifetime risk likely varies depending on the specific variant paired with A91V. Although we applied strict criteria to include only HGMD-defined PRF1 disease mutations reported in symptomatic patients with FHL2, some of these variants may exert a more profound functional impact, enhancing their penetrance when coexpressed with A91V. For example, G149S and W374∗ were more frequently observed in symptomatic individuals compared with asymptomatic ones (4 vs 1 and 4 vs 2 from different pedigrees). Other variants such as R104C, K285del, and F421C were associated with lethal outcomes but were not found in asymptomatic individuals. Conversely, 8 disease mutations (eg, G45R and D128N) were exclusively present in 10 asymptomatic UKB participants carrying A91V/pLOF genotypes. Extending the analysis to possible disease mutations, the genotype A91V/N252S was observed in 105 asymptomatic UKB participants. Additionally, 22 homozygous N252S participants without FHL2-related phenotypes were identified in the UKB, supporting its classification as a benign polymorphism.14 These observations stimulate discussion whether variants such as A91V and N252S have any role in systemic-onset juvenile idiopathic arthritis, multiple sclerosis, or acquired aplastic anemia as postulated in previous studies.10,12,44 Taken together, the incomplete penetrance of PRF1 A91V/pLOF combination indicates that the genotype cannot reliably predict disease phenotype.
Perforin expression and perforin-mediated killing assays provide valuable insights into the functional impact of individual variants.45 In our study, perforin expression in 6 patients with A91V/pLOF genotype (with low penetrance of HLH-related disease manifestations) was similar to that of 8 patients with biallelic non-A91V perforin variants of which at least 1 was hypomorphic (all presenting with symptomatic FHL2). Thus, for patients with partly reduced but not absent perforin expression, there was no consistent correlation between perforin expression and clinical outcome. Furthermore, we observed normal ex vivo NK-cell cytotoxicity in 1 symptomatic patient with A91V/pLOF genotype. These findings suggest that also functional assays are insufficient for predicting individual risk. Because family members with the same genotype can have divergent phenotypes, this problem can also not be solved by ex vivo reconstitution experiments in cell lines. Moreover, overexpression systems rarely reflect protein expression levels achieved under conditions of natural gene regulation and the coexpression of 2 different alleles at physiological levels represents an additional challenge.
As revealed by studies of patients with mixed chimerism, 20% to 30% of cells with normal cytotoxic function appear to be sufficient to protect from HLH.46 With previous estimates of PRF1 A91V providing 50% of WT perforin function,14 ∼25% of normal cytotoxic function would be expected in PRF1 A91V/pLOF carriers. Although it is unclear whether the cytotoxic activity per cell can be compared with the cytotoxic activity at the cell population level, it is interesting to note that in both situations, ∼25% of normal cytotoxicity represents the threshold at which the risk of developing HLH starts to increase significantly.
What then determines disease penetrance in individuals with A91V/pLOF genotype? One possibility is additional genetic hits including variants in other FHL genes.47-49 In most of our cases, all FHL genes were sequenced during diagnostic workup and no additional functionally validated variants were reported. Moreover, we believe that a note of caution is still justified when interpreting the existing data on genetic pathway defect accumulation in FHL. In 13 of 24 adult patients with HLH with digenic mutations including perforin variants reported by Zhang et al47 and in all 8 patients reported by Bloch et al,48 the perforin variant was A91V or N252S. As mentioned earlier, these are frequent alleles that, in heterozygosity or homozygosity, are not disease-causing and that cannot be compared with murine knockout alleles.49 Overall, the evidence for clinical relevance of pathway defect accumulation in human FHL is limited and gathered from small cohorts, calling for larger population-based studies to resolve this question. We, and others, have previously shown that HLH penetrance in humans and mice with genetic defects of cytotoxicity is defined by the genetically determined residual lytic activity of CTLs and their ability to control a trigger for disease induction,50,51 defining HLH as a threshold disease.52 It is therefore reasonable to speculate that the variability in disease manifestation in individuals with identical genotypes reflects heterogeneous exposure and control of potential triggers. In this group with a hypomorphic allele constellation, a trigger was reported in 40%. EBV was implicated as a trigger in half of the cases, whereas other infections and 3 lymphomas accounted for the remainder. Notably, EBV infection did not invariably trigger disease, with 6 individuals with A91V/pLOF genotype experiencing asymptomatic EBV seroconversion. Moreover, no disease trigger such as infection, lymphoma, or connective tissue disease was identified in the other 60% of cases, which reflects our experience in childhood FHL.4 Overall, our data indicate that A91V/pLOF is a risk genotype, especially for systemic HLH, but additional factors including identified and unnoticed infectious or malignant triggers and potentially unexplored factors such as (epi-)genetic factors, microbiome, or skewed allelic expression are required for disease manifestation. In any case, genetic factors can set the stage, supporting recommendations that also adult patients with HLH should undergo genetic testing, in particular, if no infectious, rheumatological, or malignant trigger can be identified.
Some limitations should be pointed out. Our study only captured epidemiological data on 10 of 203 known HGMD disease mutations in trans with A91V. The investigation of additional allele combinations in the future may offer additional insights. Of note, because of the age at recruitment of UKB participants, individuals who died from FHL2-related complications before age 40 years would not have been included and, as a consequence, the most deleterious A91V/pLOF genotypes could have been missed. In addition, a healthy volunteer bias has been observed in the UKB cohort,53 which might have affected recruitment of individuals with significant HLH-linked morbidity. Finally, the study primarily focused on European and US populations. Different prevalence of PRF1 genotypes in other populations and different exposure to pathogens as relevant disease triggers calls for further research to assess the significance of A91V/pLOF genotypes in diverse ethnic groups.
What are the implications of our study for the management of A91V/pLOF carriers? If seen as a group, the absolute risk for FHL2-associated disease manifestations is low, but the potential severity of FHL2 demands close monitoring of asymptomatic carriers. Of the 14 patients with a lethal outcome, 10 died during their first FHL2-related disease episode, showing that A91V/pLOF is not inherently “less severe” regarding clinical consequences once disease manifests. Certain pLOF alleles, such as G149S or W374∗, appear associated with higher risks and warrant particularly vigilant observation. Based on our findings, preemptive HSCT is not justified for all asymptomatic A91V/pLOF carriers because of the low overall disease risk balanced against significant transplantation-associated risks. However, HSCT should be considered for any individual with A91V/pLOF genotype who develops FHL2-related disease manifestations, including adult-onset cases. Further clinical experience may eventually support preemptive HSCT of specific high-risk genotypes. In any case, management of asymptomatic individuals with A91V/pLOF genotype should include patient education and regular monitoring for signs of FHL2, for example, blood counts for cytopenia, ultrasound for hepatosplenomegaly, and neurological examination.
Our study highlights the complexity of genotype-phenotype correlation in genetic disorders, also beyond immunohematological diseases. It emphasizes the importance of integrating multiple approaches, including clinical observation, population-based studies, and functional assays, to better understand the clinical implications of genetic variants. This integrative approach is particularly relevant to judge the relevance of hypomorphic variants that can not only cause typical childhood-onset but also adult-onset disease and require a better prognostic understanding at the dawn of genetic NBS.
Acknowledgments
The authors thank the Center for Chronic Immunodeficiency Advanced Diagnostics Unit and the FREEZE biobank. The authors acknowledge the Lighthouse Core Facility, funded in part by the Medical Faculty, University of Freiburg (project number: 2021/B3-Fol) and the Deutsche Forschungsgemeinschaft (project number: 450392965). This research has been conducted using the UK Biobank resource (application number 103789). Figures 1 and 2B and the visual abstract were created with BioRender.com.
This study was supported by the German Research Foundation (CRC1160 TPA01; DFG 256073931), intramural research funding from the Research Commission of the University of Freiburg, Germany, and the German Childhood Cancer Foundation (DKS2018.11 and DKS2024.04). O.W. is fellow of the IMM-PACT Programme for Clinician Scientists, Department of Medicine II, Medical Center, University of Freiburg and Faculty of Medicine, University of Freiburg, funded by the Deutsche Forschungsgemeinschaft (German Research Foundation, 413517907).
Authorship
Contribution: O.W. compiled the clinical cohort, performed literature searches, performed functional assays, analyzed data, arranged figures, and wrote the manuscript; O.W., O.B., and A.K. performed population genetic analyses in the UK Biobank; E.S., F.O., J.M., M.L.C., A.C., L.B., K.G., G.d.S.B., W.Z., R.M., and L.H. performed functional assays and genetic testing; E.S., F.P., K.G., D.M., R.M., C.D.F., K.W., F.T., C.M., E.G., S.C., S.G., K.L., and S.E. provided patient care and clinical information on individuals with A91V/pLOF genotype; S.E. contributed to project development, supervised the work, and revised the manuscript; and all authors approved the final submitted version.
Conflict-of-interest disclosure: R.M. is employed part-time by Pharming Healthcare. The remaining authors declare no competing financial interests.
Correspondence: Oliver Wegehaupt, University Medical Center Freiburg, Center for Chronic Immunodeficiency, Institute for Immunodeficiency, Breisacher Str 115, 79106 Freiburg, Germany; email: oliver.wegehaupt@uniklinik-freiburg.de; and Stephan Ehl, University Medical Center Freiburg, Center for Chronic Immunodeficiency, Institute for Immunodeficiency, Breisacher Str 115, 79106 Freiburg, Germany; email: stephan.ehl@uniklinik-freiburg.de.
References
Author notes
The data sets generated during this study are available upon reasonable request from the corresponding authors, Oliver Wegehaupt (oliver.wegehaupt@uniklinik-freiburg.de) and Stephan Ehl (stephan.ehl@uniklinik-freiburg.de).
The online version of this article contains a data supplement.
There is a Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.