In this issue of Blood, Wegehaupt and colleagues explore the challenges in predicting disease risk in carriers of biallelic perforin (PRF1) gene variants (see figure).1
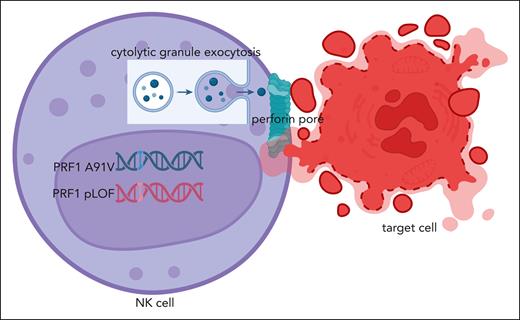
Perforin-mediated cytolysis. An NK cell possessing the PRF1 A91V/pLOF genotype is activated upon recognition of its target cell, resulting in cytolytic granule exocytosis. Perforin is released along with granzyme to the immunologic synapse between the cells for perforin to form a pore/channel between the cells, which allows for for granzyme delivery triggering apoptosis of the target cell. Figure created with BioRender via subscription to the University of Alabama at Birmingham.
Perforin-mediated cytolysis. An NK cell possessing the PRF1 A91V/pLOF genotype is activated upon recognition of its target cell, resulting in cytolytic granule exocytosis. Perforin is released along with granzyme to the immunologic synapse between the cells for perforin to form a pore/channel between the cells, which allows for for granzyme delivery triggering apoptosis of the target cell. Figure created with BioRender via subscription to the University of Alabama at Birmingham.
Biallelic pathogenic variants in PRF1 were the first identified genetic etiology of familial hemophagocytic lymphohistiocytosis (FHL),2 a highly fatal hyperinflammatory disorder typically presenting in infancy.3 Perforin is responsible for creating a channel/pore (punching a hole) between cytolytic lymphocytes, natural killer cells and CD8 T cells, and their target cells, allowing for the delivery of apoptosis-inducing granzyme proteins (see figure).3 The perforin pore can be disrupted by loss-of-function (LOF) PRF1 mutations resulting in absent2 or even delayed killing of target cells, thus prolonging the interaction of the cytolytic lymphocyte and its antigen-presenting target cell.4 The resulting prolonged engagement between the defective cytolytic lymphocyte and its target cell results in excess proinflammatory cytokine production believed responsible for the subsequent multiorgan dysfunction and associated morbidity and mortality of FHL. FHL can also result from biallelic defects in several cytolytic pathway genes (eg, UNC13D, STXBP2, STX11, RAB27A) upstream of perforin that are required for delivery of perforin- and granzyme-containing cytolytic granules to the immunologic synapse between cytolytic lymphocytes and their target cells.3,PRF1 mutations are a relatively common cause of FHL among those of European descent and African heritage. One well-studied common (∼8% of Europeans) hypomorphic PRF1 missense variant (c.272C>T, p.A91V) impairs cytolytic function up to 50%.5 However, this particular polymorphism is not believed to be a risk allele for FHL when present as a homozygous variant.6 In their current report, Wegehaupt et al explore the contribution of PRF1 p.A91V to FHL in trans with other predicted LOF (pLOF) PRF1 variants.
The authors studied clinical and functional data from 52 FHL patients with A91V/pLOF PRF1 variants from an FHL network registry (associated with the Histiocyte Society), where pLOF was defined as “disease mutation” in the Human Gene Mutation Database. Thirty-nine (72%) patients (mean age of onset 20 years) from this cohort manifested FHL, with 4 having recurrent disease, and 14 (27%) suffering death. Family screening identified an additional 14 family members (mean age 29 years) with A91V/pLOF PRF1, and only 1 was symptomatic. In addition, 21 A91V/pLOF PRF1 carriers, including 12 with genotypes identical to those in the registry, were identified among 200 000 participants in the United Kingdom Biobank with none developing FHL by 73 years of age. Clearly, A91V/pLOF PRF1 carriers are not all destined to develop FHL, and, even if they do, it frequently presents well beyond infancy. Hemophagocytic lymphohistiocytosis (HLH) presenting beyond infancy is often termed secondary HLH (sHLH), and has been associated with heterozygous, often missense, variants in FHL genes, including PRF1.3 There are likely environmental and disease triggers (eg, infection, lymphoma, autoinflammatory disease) that contribute to HLH disease manifestation in the context of a threshold model of disease.7
Heterozygous A91V PRF1 mutations have been identified in fatal cases of H1N1 influenza sHLH,5 and, compared with controls, increased percentages of rare heterozygous missense PRF1 variants have been noted in children with systemic juvenile idiopathic arthritis,8 particularly in those who develop sHLH.9 It is unclear why the penetrance of various PRF1 variants is so variable. Similarly, both fathers (who shared the same mutation with their offspring) of 2 unrelated teenagers with sHLH associated with an identical rare missense mutation in the FHL gene, RAB27A, have never experienced sHLH.10 In addition to associated triggers, the degree of penetrance is likely related to the severity of the defect of the various PRF1 variants. Indeed, Wegehaupt and colleagues noted PRF1 variants that were the most deleterious (nonsense, deletions, frameshift leading to premature stop codons) were found to be enriched in symptomatic (38%) compared with asymptomatic (12%) A91V/pLOF PRF1 carriers. Moreover, the United Kingdom Biobank reveals that approximately 1 in 1000 people in the general population possess PRF1 A91V paired with a definite or probable pLOF PRF1 variant.
Extrapolating the numbers of A91V/pLOF PRF1 carriers just described suggests that over 600 newborns annually in Germany, the United Kingdom, or France may possess such a genotype. This begs the question of whether to use newborn genetic screening to identify potential PRF1 FHL individuals who may benefit from hematopoietic stem cell transplant prior to disease manifestation, when outcomes are less optimistic. However, functional testing (intracellular perforin expression levels and cytolytic capacity) of lymphocytes from individuals with various A91V/pLOF PRF1 variants does not correlate function with FHL development. Neither intracellular perforin levels nor natural killer cell cytotoxicity can predict FHL disease risk in individuals with the A91V/pLOF PRF1 genotype. So, for now, early screening for FHL development by serum ferritin (a highly sensitive marker for FHL and sHLH)3 in the setting of fever is appropriate for those individuals identified with the A91V/pLOF PRF1 genotype.
Conflict-of-interest disclosure: R.Q.C. is coprincipal investigator in an investigator-initiated clinical trial exploring anakinra treatment for severe COVID-19, supported in part by Sobi; received consulting fees from Sobi, Novartis, Neurogene, AS2 Biotherapeutics, AbbVie, Pfizer, and CareerPhysician; receives textbook royalties from Springer and editorial income from ACR Open Rheumatology; and is a paid question writer for the American Board of Pediatrics.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal