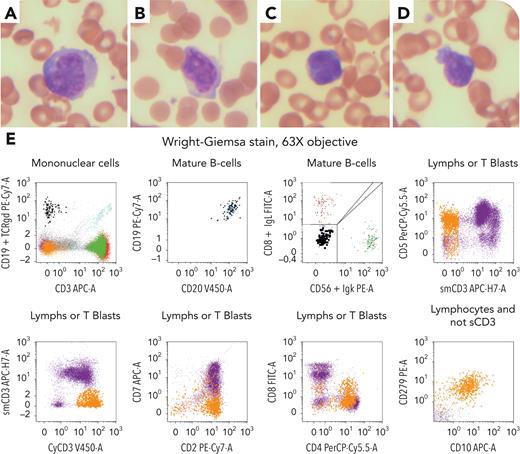
A 74-year-old woman presented with fever, weight loss, diffuse lymphadenopathy, and splenomegaly. Laboratory results were as follows: hemoglobin 85 g/L, white blood cells 3.83 × 109/L, platelets 106 × 109/L, creatinine 81 mmol/L, and lactate dehydrogenase 424 IU. Peripheral blood (PB) smear showed a few large- and medium-sized abnormal lymphoid cells with irregular nuclear contour with or without prominent nucleoli (panels A-D: original magnification ×600; Wright-Giemsa stain). Flow cytometry (panel E) detected 2 abnormal populations. One population (∼1%) expressed CD19 and CD20 (bright), was negative for CD5, CD10, and CD79b, without light chain expression, consistent with circulating B-lymphoproliferative disorder. The other population (∼5%) expressed CD2, cytoplasmic CD3, CD4, CD5, CD10, and CD279, and was negative for CD1a, CD3, CD7, CD34, CD99, and terminal deoxynucleotidyltransferase, in keeping with features of T-follicular helper cells. Polymerase chain reaction study of PB was positive for immunoglobulins H and K as well as T-cell receptor-β and -γ rearrangements. Interventional radiology guided biopsy on the left supraclavicular lymph node confirmed the presence of Epstein-Barr virus–positive diffuse large B-cell lymphoma and nodular T-follicular helper lymphoma, angioimmunoblastic type. Fluorescence in situ hybridization for MYC and BCL2 rearrangement was negative. Bone marrow aspirate and biopsy revealed no overt lymphoma involvement.
Composite lymphoma, an uncommon type of lymphoid malignancy, is typically diagnosed based on tissue biopsies. This case uniquely demonstrated a manifestation of a composite B- and T-lymphoproliferative disorder in PB, which can be easily overlooked. It highlights the importance of careful assessment of PB cell morphology and using appropriate ancillary studies.
For additional images, visit the ASH Image Bank, a reference and teaching tool that is continually updated with new atlas and case study images. For more information, visit https://imagebank.hematology.org.