TO THE EDITOR:
We read with interest the recent publication by van Outersterp et al1 describing the differential sensitivity of acute lymphoblastic leukemia (ALL) ABL-class gene fusions to adenosine triphosphate (ATP)–competitive tyrosine kinase inhibitors (TKIs). The aforementioned study investigated the efficacy of imatinib, dasatinib, and bosutinib in cell lines harboring ABL1 (n = 7), ABL2 (n = 1), PDGFRB (n = 11), and CSF1R (n = 2) gene fusions and reported that lack of Src homology 3 (SH3) domain does not confer resistance to TKIs. Here, we report additional data for the efficacy of the TKIs nilotinib and ponatinib, as well as the myristate pocket–targeted allosteric inhibitor asciminib, against ABL1 (n = 5) and ABL2 (n = 3) gene fusions, collectively referred to as ABL rearrangements (ABLrs).
Philadelphia (Ph)-like ALL is associated with high rates of treatment failure and relapse.2,3 TKIs such as dasatinib and ponatinib effectively target BCR::ABL1+ (Ph+) ALL and their therapeutic utility is being examined in clinical trials for ABL-class Ph-like ALL (NCT03564470, NCT06124157, and NCT02779283). Resistance to the ATP-competitive TKIs imatinib and dasatinib is well established in the clinical setting,4 and a novel and therapeutically targetable binding pocket within the ABL1 kinase was subsequently identified to overcome this resistance.5,6 This myristate binding pocket, located toward the C-terminus of the ABL1 kinase domain, becomes an allosteric negative regulator of ABL1 upon binding of the N-terminus myristoyl moiety.7 Because ABLr leukemias typically involve the truncation of N-terminus exons, this autoinhibitory regulation is lost. Asciminib was rationally designed to potently bind in the myristate pocket of the BCR:ABL1 kinase, and once bound, 1 of the 4 α-helices that form the myristate pocket (helix αΙ) undergoes conformational changes resulting in a ∼90° bend to form helix αΙ’. Helix αΙ’ acts as a docking site for the SH2 domain and, due to the tight coupling of the SH2 and SH3 domains, facilitates docking of the SH3 domain to the SH2-kinase linker region and the ATP pocket of the kinase domain.6-9 Combined, these interactions lock ABL1 in an inactive conformation. Due to the homology between ABL1 and ABL-related gene (ARG, protein encoded by the ABL2 gene), including conservation of the amino acids that comprise the myristate binding pocket and the presence of a myristoyl moiety in ARG,7 it is hypothesized that asciminib may target ABL2 rearrangements. In vitro and in vivo studies have demonstrated synergy when asciminib is used in combination with TKIs,9-12 and asciminib-TKI combination clinical trials have recently concluded (NCT02081378). Importantly, phase 1 trials involving chronic myeloid leukemia (CML) and patients with Ph+ ALL have demonstrated durable molecular responses in some patients receiving asciminib after intolerance or resistance to ≥2 TKIs, including in cases of TKI resistance via the ABL1 p.T315I gatekeeper mutation.4,9 Although asciminib effectively targets both the e13a2 and e14a2 BCR::ABL1 fusion proteins present in most cases of CML, as well as the e1a2 variant observed more commonly in Ph+ ALL, there are limited data for its efficacy against Ph-like ALL harboring ABL1 and ABL2 gene rearrangements.13 Asciminib could provide a new treatment avenue for these high-risk patients, particularly in the event of frontline TKI resistance development, and evaluation of asciminib efficacy against ABLrs is the basis of this study.
More than 20 different ABLrs have been identified in patients with ALL,14,15 and we selected a subset encompassing various ABL1/ABL2 breakpoints for our investigations (Figure 1). Transduced Ba/F3 cell lines harboring plasmids expressing each ABLr were generated as described in the supplemental Methods, available on the Blood website. After confirmation of cytokine (interleukin-3 [IL-3]) independence, annexin V/7-aminoactinomycin D (7-AAD) cytotoxicity and intracellular phosphoflow analyses of STAT5/CRKL activation were used to determine asciminib efficacy in vitro. The lethal dose of inhibitor required for 50% cell death (LD50) was calculated and compared with the clinically achievable drug concentrations. The steady state maximal plasma concentrations for nilotinib (400 mg twice per day, patients with CML) and ponatinib (45 mg per day, patients with CML) are 3.6 μM and 145 nM, respectively,16,17 whereas asciminib ranges from ∼2 μM (40 mg twice per day, patients with chronic-phase CML)4 to ∼7.2 μM (200 mg twice per day, patients with CML with the ABL1 p.T315I mutation).18
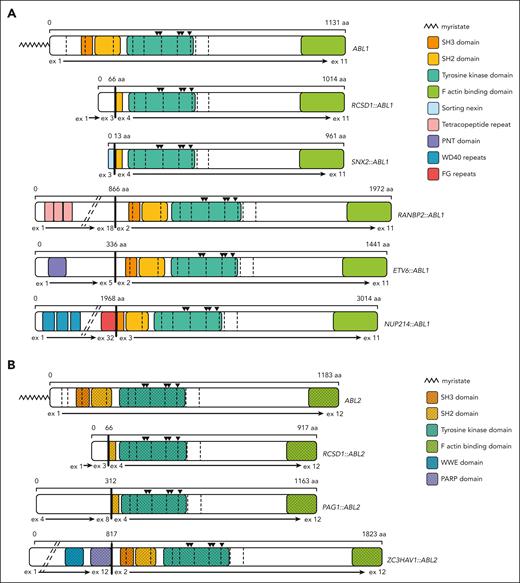
Schematics depicting the functional domains and breakpoints for ABL1 and ABL2 fusion genes. In all fusion genes, the ABL1 (A) and ABL2 (B) tyrosine kinase domains are retained and the autoinhibitory myristate moieties are truncated. The residues required for formation of the myristate binding pocket are retained in all fusions as indicated by black triangles above the kinase domain. Fusion of different 5’ partner genes results in retention (breakpoint at exon 2), truncation (breakpoint at exon 3), or deletion (breakpoint at exon 4) of the ABL1/ABL2 SH3 domain. Breakpoints are denoted by vertical bold black lines and exons by vertical dashed lines. Diagonal dashed lines indicate unstructured regions of fusion partner genes that have been omitted for simplicity. The following isoforms were used for schematic construction: ABL1 NM_007313 and ABL2 NM_001168238.
Schematics depicting the functional domains and breakpoints for ABL1 and ABL2 fusion genes. In all fusion genes, the ABL1 (A) and ABL2 (B) tyrosine kinase domains are retained and the autoinhibitory myristate moieties are truncated. The residues required for formation of the myristate binding pocket are retained in all fusions as indicated by black triangles above the kinase domain. Fusion of different 5’ partner genes results in retention (breakpoint at exon 2), truncation (breakpoint at exon 3), or deletion (breakpoint at exon 4) of the ABL1/ABL2 SH3 domain. Breakpoints are denoted by vertical bold black lines and exons by vertical dashed lines. Diagonal dashed lines indicate unstructured regions of fusion partner genes that have been omitted for simplicity. The following isoforms were used for schematic construction: ABL1 NM_007313 and ABL2 NM_001168238.
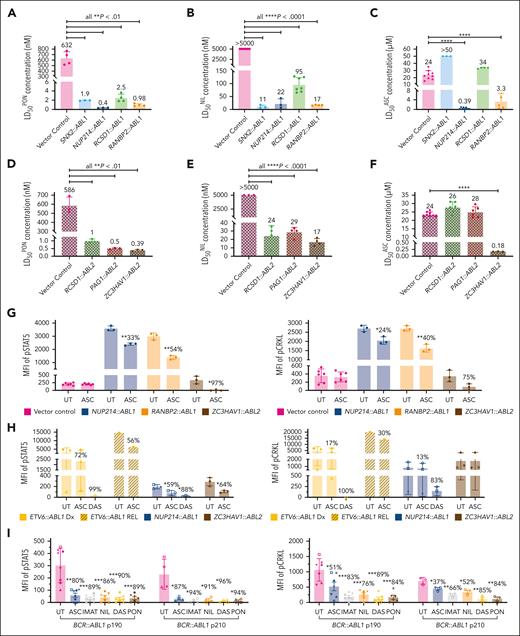
Ponatinib is approved for the treatment of Ph+ ALL,17 and we observed preclinical efficacy of both ponatinib and nilotinib against all ABLrs investigated in this study (n = 8). Ba/F3 cells harboring ABL1r demonstrated LD50 concentrations for both ponatinib (LD50PON) and nilotinib (LD50NIL) in the low nanomolar range (LD50PON ≤ 2.5 nM [P < .01] and LD50NIL < 95 nM [P < .0001] for all fusions vs control cells). Conversely, vector control cells demonstrated LD50 concentrations exceeding the clinically achievable dose for both TKIs (LD50PON = 586 nM; LD50NIL > 5 μM). Cells harboring ABL2rs also demonstrated LD50 concentrations for both TKIs in the low nanomolar range (LD50PON ≤ 1 nM [P < .01] and LD50NIL < 29 nM [P < .0001] for all fusions vs control cells; Figure 2A-B, D-E).
Asciminib demonstrates varying efficacy against ABL1 and ABL2 fusion genes in vitro. In vitro targeting of ABL1 and ABL2 fusion genes with ponatinib (A,D), nilotinib (B,E), and asciminib (C,F). (A-F) LD50 concentrations from cell death assays targeting different ABL1 and ABL2 fusion genes identified in ALL. LD50 concentrations are from a minimum biological replicate of 3. (G-I) Intracellular flow cytometry of transduced Ba/F3 cells or patient blast cells and human CD45+ patient-derived xenograft (PDX) cells treated with asciminib (5 μM), imatinib (5 μM), nilotinib (1 μM), dasatinib (100 nM), or ponatinib (100 nM) for 2 to 6 hours and stained with antibodies for pSTAT5 and pCRKL. Untreated cells were used as controls. Percentage reductions in the presence of asciminib and TKIs (where cell number allowed) are specified. For the transduced cell lines, 3 biological replicates were performed. For patient and PDX samples, different symbols delineate individual patients. Columns represent mean ± standard deviation error bars with individual data points shown. Statistics were calculated by Student t test and significance is denoted with asterisks ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001. DAS, dasatinib; IMAT, imatinib; MFI, mean fluorescence intensity.
Asciminib demonstrates varying efficacy against ABL1 and ABL2 fusion genes in vitro. In vitro targeting of ABL1 and ABL2 fusion genes with ponatinib (A,D), nilotinib (B,E), and asciminib (C,F). (A-F) LD50 concentrations from cell death assays targeting different ABL1 and ABL2 fusion genes identified in ALL. LD50 concentrations are from a minimum biological replicate of 3. (G-I) Intracellular flow cytometry of transduced Ba/F3 cells or patient blast cells and human CD45+ patient-derived xenograft (PDX) cells treated with asciminib (5 μM), imatinib (5 μM), nilotinib (1 μM), dasatinib (100 nM), or ponatinib (100 nM) for 2 to 6 hours and stained with antibodies for pSTAT5 and pCRKL. Untreated cells were used as controls. Percentage reductions in the presence of asciminib and TKIs (where cell number allowed) are specified. For the transduced cell lines, 3 biological replicates were performed. For patient and PDX samples, different symbols delineate individual patients. Columns represent mean ± standard deviation error bars with individual data points shown. Statistics were calculated by Student t test and significance is denoted with asterisks ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001. DAS, dasatinib; IMAT, imatinib; MFI, mean fluorescence intensity.
We next examined asciminib sensitivity, establishing efficacy in 3 of 5 investigated ABL1 fusion proteins. Ba/F3 NUP214::ABL1 cells were sensitive to asciminib (LD50 of asciminib [LD50ASC] = 0.39 μM), whereas Ba/F3 vector control cells were insensitive (LD50ASC = 24 μM; P < .0001; Figure 2C). Similarly, Ba/F3 RANBP2::ABL1 cells demonstrated asciminib sensitivity within the clinically achievable range (LD50ASC = 3.3 μM; P < .0001 vs control cells). Conversely, Ba/F3 cells harboring SNX2::ABL1 and RCSD1::ABL1 gene fusions were resistant to asciminib with inhibitory concentrations well above the clinically achievable dose (LD50ASC = 50 μM and 34 μM, respectively). We also evaluated asciminib efficacy in Ba/F3 cells harboring various ABL2 fusion genes, confirming sensitivity in 1 of 3 fusions investigated (Figure 2F). Ba/F3 RCSD1::ABL2 and PAG1::ABL2 cells were resistant to asciminib (LD50ASC = 26 μM and 28 μM); however, Ba/F3 ZC3HAV1::ABL2 cells were sensitive to asciminib with an inhibitory concentration in the nanomolar range (LD50ASC = 0.18 μM; P < .001 vs control cells). Additionally, we confirmed asciminib-mediated inhibition of kinase signaling in each of the transduced cell lines that demonstrated asciminib sensitivity by cell death assay (Figure 2G), as well as in blasts cells harvested from NUP214::ABL1 patient-derived xenografts (n = 3) and patients harboring the ZC3HAV1::ABL2 fusion (n = 3). We also established asciminib-mediated kinase inhibition in the blast cells of ETV6::ABL1 patients (n = 4), further expanding the number of asciminib-sensitive ABL1 fusion genes identified (Figure 2H). Furthermore, when asciminib-mediated kinase inhibition was examined in patients with BCR::ABL1+ ALL, we observed a significant decrease in activated (phosphorylated) signal transducer and activator of transcription 5 (STAT5) (P < .01) and Crk-like protein (CRKL) (P < .05) in patients harboring both p190 and p210 BCR::ABL1 isoforms (Figure 2I). Importantly, the reduction of pSTAT5 and pCRKL in response to asciminib treatment was comparable with that observed for TKIs.
This study evaluated the efficacy of TKIs ponatinib and nilotinib and the novel allosteric ABL inhibitor asciminib against various ABLrs identified in Ph-like ALL. Although all fusions demonstrated sensitivity to ponatinib and nilotinib, asciminib efficacy varied between the 5 ABL1 fusions and 3 ABL2 fusions investigated. Importantly, we report for the first time, to our knowledge, that asciminib is efficacious against specific ABL2-rearranged ALL in vitro. Interrogation of the ABL1/ABL2 breakpoints (Figure 1) highlighted the retention or deletion of the SH3 domain. Similar to what was observed for imatinib, dasatinib, and bosutinib,1 our results indicated that absence of the SH3 domain did not influence sensitivity to ponatinib or nilotinib in vitro. We now highlight the importance of the SH3 domain for asciminib sensitivity. Our preclinical testing demonstrated asciminib efficacy in cells harboring the NUP214::ABL1, RANBP2::ABL1, ETV6::ABL1, and ZC3HAV1::ABL2 gene fusions, in which ABL1/ABL2 breakpoints result in retention of the SH3 domain. Conversely, cells harboring the SNX2::ABL1, RCSD1::ABL1, PAG1::ABL2, and RCSD1::ABL2 gene fusions were not sensitive to asciminib and lack the SH3 domain. Significantly, these data indicate that there is a critical region of the SH3 domain required for effective asciminib binding and kinase inhibition. We propose that interrogation of ABLr patient breakpoints to determine the inclusion/exclusion of the SH3 domain could be used as a surrogate test, in conjunction with in vitro kinase sensitivity assays, for predicting whether a patient is likely to respond to asciminib treatment. Further studies evaluating asciminib efficacy in xenograft models and in vitro studies identifying the key sections of the SH3 domain essential for asciminib sensitivity are required and form our current research endeavors.
ALL patient and patient-derived xenograft samples stored in our liquid nitrogen biobank were used for phosphoflow assays. Patients provided written informed consent for sample collection and storage for future ethically approved research including the studies presented here. Research involving human samples was approved by the Women's and Children's Health Network Human Research Ethics Committee. Patient-derived xenografts were established and maintained on a protocol approved by the South Australian Health and Medical Research Institute (SAHMRI) Animal Ethics Committee.
Acknowledgments
This research was undertaken with the following financial and other support to L.N.E.: the Cancer Council SA's Peter Nelson Leukaemia Research Fellowship on behalf of its donors (ID# 022212); the Channel 7 Children's Research Foundation (ID #171412); and the Cancer Council SA's Beat Cancer Project on behalf of its donors and the State Government through the Department of Health (ID# 22234). E.L. is supported by a University of Adelaide Research Scholarship.
Authorship
Contribution: L.N.E. designed the study, performed scientific studies, analyzed the data, wrote the manuscript, and created the figures; E.L. designed the study, performed scientific studies, and analyzed the data; E.C.P. designed the study and wrote the manuscript; C.E.S. performed scientific studies and analyzed the data; S.L.H. performed scientific studies and analyzed the data; B.J.M. performed scientific studies and analyzed the data; M.O.F. performed scientific studies and analyzed the data; D.T.Y. designed the study and provided scientific and clinical insight; T.P.H. designed the study and provided scientific and clinical insight.; D.L.W. designed the study and provided scientific insight; and all authors critically reviewed the manuscript.
Conflict-of-interest disclosure: D.T.Y. declares honoraria, membership on an entity's board of directors or advisory committees, and research funding from Novartis; honoraria and membership on an entity's board of directors or advisory committees from Pfizer; honoraria, membership on an entity's board of directors or advisory committees, and research funding from Amgen; honoraria and membership on an entity's board of directors or advisory committees from Takeda; and research funding from Bristol Myers Squibb. T.P.H. declares consultancy, honoraria, and research funding from Novartis; and consultancy and research funding from Bristol Myers Squibb and Ariad. The remaining authors declare no competing financial interests.
Correspondence: Deborah White, South Australian Health and Medical Research Institute, North Terrace, Adelaide 5000, Australia; email: deborah.white@sahmri.com.
References
Author notes
L.N.E. and E.L. are joint first authors.
Data are available from the corresponding author, Deborah L. White (deborah.white@sahmri.com), on request.
The online version of this article contains a data supplement.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal