In this issue of Blood, Leyte-Vidal et al use cell-viability assays, molecular simulations, and drug-binding studies to demonstrate that BCR::ABL1 N-lobe mutants, such as M244V, confer resistance to asciminib, adding another potential mechanism of resistance to this drug.1
Despite the improved outcomes obtained with tyrosine kinase inhibitors (TKIs), about 20% to 30% of chronic-phase chronic myeloid leukemia patients still experience refractoriness and/or relapse.2 New treatment strategies are needed to overcome the common mechanisms of TKI failure and avoid potential long-term off-target effects of second- and third-generation drugs.3 Asciminib is the first allosteric inhibitor and was recently approved for patients who previously failed 2 lines of treatment because of resistance and/or intolerance. In the United States, but not in Europe, asciminib can also be prescribed for patients with the T315I mutatation. Asciminib acts on the myristoyl pocket in the C lobe and not on the adenosine triphosphate (ATP)-binding site. During physiological conditions, ABL1 is inhibited by the myristoylated N-terminus peptide that acts by inducing the “capping” phenomenon, the juxtaposition of the SH subunits to the kinase, which is an inactive conformation. This mechanism of regulation is lost in the BCR::ABL1 fusion gene, leaving the kinase in a constitutional active form. Asciminib mimics the role of the peptide by restoring the inactive kinase conformation.4 Asciminib may be combined with all available ATP-competitive TKIs. A recent report with a median 5.9-year follow-up of a phase 1a trial conducted in a group of patients with chronic-phase chronic myeloid leukemia who were heavily pretreated (71.3% treated previously with ≥3 TKIs) showed that 70 (60.9%) were still receiving treatment. The MR3(BCR::ABL1 ratio ≤0.1% international scale [IS]) rate was 65%, the MR4 (BCR::ABL1 ratio ≤0.01% IS) rate was 23.6%, and the MR4.5 (BCR::ABL1 ratio ≤0.0032% IS) rate was 18.9%. The final dose for patients without the T315I mutation was set at 40 mg twice daily, and for those with the T315I mutation the dose was escalated to 200 mg twice daily. Adverse events recorded were generally manageable, with the most common being arthralgia, fatigue, increased amylase/lipase level, and thrombocytopenia.5 The final analysis with almost 4 years of follow-up of the phase 3 ASCEMBL trial, which compared asciminib with bosutinib as third-line treatment, showed the persistence of a higher MR3 response rate in asciminib-treated patients (33.8% vs 10.5%), with a good tolerability profile.6
Potential mechanisms of resistance to asciminib have been reported previously, with the 2 main categories identified as upregulation of the ABCG2 efflux pump, resulting in low intracellular asciminib levels, and emergence of BCR::ABL1 mutations at the myristoyl binding site.7
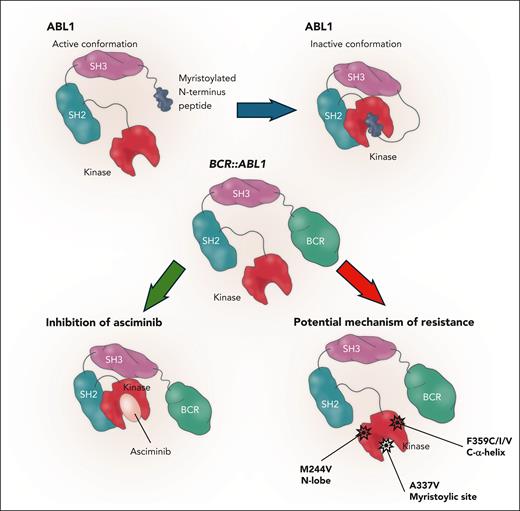
Recent data have shown mutations that reduce sensitivity to asciminib. In particular, the F359C/I/V mutation located beneath the C helix and before the A loop, with conformation depending by the state of activation of the kinase, has been described. Even if the mutation is not situated in proximity to the myristoyl pocket, it has been hypothesized that this mutation results in a shift to the active state of the kinase, with reduced affinity to asciminib.8 The A337V/T mutation is an example of a specific mutation observed after asciminib treatment. It is located at the start of the E helix and limits the capping phenomenon, which affects the difficult docking of SH subunits to the kinase domain.8 The I502L mutation is situated in the myristoyl-binding site and has been reported as a possible mutation causing insensitivity to asciminib, even though it does not impact the binding of the drug (see figure).8
Mechanism of action of asciminib and specific mutations responsible for resistance. A337V situated on myristoylic site M244V on the N lobe and F359C/I/V on the alpha helix.
Mechanism of action of asciminib and specific mutations responsible for resistance. A337V situated on myristoylic site M244V on the N lobe and F359C/I/V on the alpha helix.
Leyte-Vidal et al present findings from 4 patients resistant to asciminib, all with the M244V N-lobe mutation. Cell-viability assays were performed showing that this mutation causes about 2 logs of resistance to asciminib, which is about 1-log greater resistance than that seen in patients with the T315I mutation. Several other mutations not previously studied were included in the investigation. Mutations causing high resistance included M244V, L248V, and F317L, whereas complete resistance was seen with A337V and F359V. The A337V mutation directly impacted the myristoyl-binding site. The authors also found that the common M244V and the F359C/I/V mutations may affect the sensitivity to asciminib through a conformational change of the dock of the SH2 domain to the N lobe, not affecting directly the myristoyl-binding site but altering the allosteric inhibitory mechanism of the drug. This result highlights the importance of performing a mutational assessment before switching to asciminib. In cases of resistance to the drug, clinicians should search broadly for mutations and not just those involving the specific myristoylic site. The good news is that all these mutations (including the myristoyl-binding site mutations) can be rescued by second- and third-generation TKIs. Larger cohorts of drug-resistant patients are needed to confirm the real-life rate of resistance to asciminib.
Conflict-of-interest disclosure: M.B. has received honoraria from Novartis, Pfizer, Bristol Myers Squibb, Incyte, AOP, and AbbVie.