In this issue of Blood, McMurray et al1 describe the implementation of a standardized approach for the screening and diagnosis of systemic mastocytosis (SM) using an international health system–wide screening registry. The objective of McMurray et al’s study was to improve the accuracy of SM diagnosis by increasing the number of screened and diagnosed patients and by defining the type of examination that should be performed based on the clinical presentation.
SM is characterized by an accumulation of atypical mast cells in 1 or more internal organs.2 In adults, the most common variant (as defined by the World Health Organization) is nonadvanced SM (non-AdvSM), which includes bone marrow mastocytosis (BMM), indolent SM (ISM), and smoldering SM.3,4 Diagnosis of ISM is relatively easy because this variant typically involves skin lesions (mostly in the form of maculopapular cutaneous mastocytosis [MPCM]). This is not the case for BMM, which is therefore more difficult for a physician to spot and diagnose.5 However, it is essential to diagnose non-AdvSM because this condition worsens the patient’s quality of life and has a number of potentially life-threatening complications (such as multiple osteoporotic fractures and anaphylactic shock).
Nonspecialist physicians may not be familiar with diagnostic work for SM (consisting of specific mastocytosis-focused history and clinical, biochemical, and molecular analyses) that might reduce the likelihood of diagnosis. When mastocytosis is suspected, the analysis most frequently requested by physicians is the serum tryptase test. However, it is now clear that this test has several limitations, including sensitivity problems. Indeed, a significant proportion of patients with non-AdvSM have a normal basal serum tryptase (BST) level.6 Specificity is also a problem because an elevated BST level is associated with a genetic trait called hereditary α tryptasemia (HαT), found in 5% to 6% of the Western population.7 The KIT pD816V mutation is the hallmark of SM and is present in up to 90% of cases. Until recently, this mutation was detected with low-sensitivity techniques (such as next-generation sequencing and Sanger sequencing), which resulted in a high false-negative rate. The mutation is now detected using highly sensitive techniques, like droplet digital polymerase chain reaction (ddPCR) and allele-specific oligonucleotide real-time quantitative PCR.8 However, not all physicians have access to these techniques. Furthermore, anaphylaxis is a common presenting manifestation of SM and often prompts screening for SM. Therefore, several expert groups (including the Spanish Network on Mastocytosis [REMA]) have developed predictive scores to help physicians to screen for SM9 in patients presenting with anaphylaxis. However, not all physicians are familiar with the REMA score, and researchers have yet to establish the real-world value of the combination of this score with the BST level based upon tryptase genotype for the diagnosis of SM.
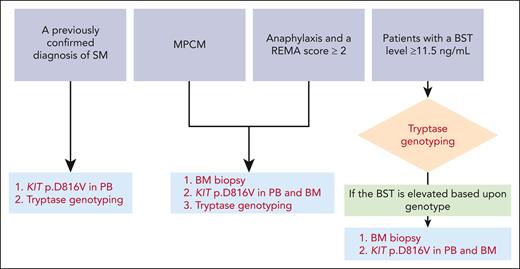
In view of the factors presented here, a comprehensive approach to the diagnosis of SM (based on simple algorithms that encompass clinical and laboratory variables) is urgently needed. By using an international health system–wide screening registry, McMurray et al have developed a standardized approach for SM screening. Over a 2-year period, the investigators compared the rates of SM diagnosis before and after standardizing care in a real-life setting. The patients included in McMurray et al’s study had either (1) a previously confirmed diagnosis of SM, (2) adult-onset monomorphic MPCM, (3) a history of anaphylaxis (with a REMA score ≥ 2) or (4) a BST level ≥ 11.5 ng/mL and were referred to a center of excellence for a clinical evaluation. The patient management scheme (depending on the inclusion criteria) is summarized in the figure.
Diagnostic workflows for SM, as suggested by McMurray et al. The presenting conditions where the diagnosis of SM should be evaluated are at the top of the figure. The additional tests needed to confirm or rule out a diagnosis of SM are shown in red at the bottom of the figure. PB and BM samples should be screened for the KIT pD816V mutation with highly sensitive techniques, such as droplet digital PCR and allele-specific oligonucleotide real-time quantitative PCR. PB, peripheral blood.
Diagnostic workflows for SM, as suggested by McMurray et al. The presenting conditions where the diagnosis of SM should be evaluated are at the top of the figure. The additional tests needed to confirm or rule out a diagnosis of SM are shown in red at the bottom of the figure. PB and BM samples should be screened for the KIT pD816V mutation with highly sensitive techniques, such as droplet digital PCR and allele-specific oligonucleotide real-time quantitative PCR. PB, peripheral blood.
Out of a population of 2 752 884 beneficiaries, 249 were included in the study. Only 47 of the 249 individuals had already been diagnosed with SM as of 1 July 2021, whereas almost twice as many (94) were diagnosed with SM using the standardized approach for SM screening, as of 1 July 2023. In the absence of this standardized approach, nearly half of the SM diagnoses would have been missed. Interestingly, the mean BST level was lower for patients diagnosed using the standardized approach (19.3 ng/mL vs 50.2 ng/mL before standardization; P < .0001), and the proportion of SM cases with a normal BST level was 4.3% before standardization and 14.9% after standardization. The most accurate SM screening tests were an elevated BST level based upon tryptase genotype (sensitivity: 84.2%; specificity: 90.2%), the presence of monomorphic MPCM (sensitivity: 63.8%; specificity: 97.6%), and the combination of a REMA score ≥2 and an elevated BST level based upon tryptase genotype (sensitivity: 30.3%; specificity: 97.6%). Interestingly, an elevated BST level alone (>20 ng/mL, without considering the tryptase genotype) had a sensitivity of only 59.6% and a specificity of only 71.4%. The investigators astutely addressed the reasons for missed SM diagnoses. For example, the reasons why a BM examination was not performed included a BST level considered insufficiently elevated, failure to calculate the REMA score, and failure to detect KIT p.D816V in peripheral blood (due notably to the use of low-sensitivity techniques). Moreover, erroneous immunohistochemical analyses were noted for 10 of the 11 patients with a missed diagnosis and a BM biopsy, showing why review by hematopathologists with experience with SM is important. Lastly, the use of TPSAB1 genotyping results to interpret the BST level helped to reduce the need for BM examinations in a large number of individuals with HαT but no other signs of SM.
Although SM is a rare disease, it is becoming more frequently diagnosed with population estimates of 1 in 10 000 to 1 in 20 000 now increased to 1 in 5000.10 This increase in prevalence is most likely related to higher diagnosis rates due to improved recognition by physicians (eg, the use of diagnostic algorithms in cases of anaphylaxis and more accurate hematopathological assessment) and greater access to new diagnostic tools such as ddPCR. However, the diagnostic workup for SM still varies greatly from one hospital to another. We expect that the implementation of McMurray et al’s standardized approach, specifically intended for non-AdvSM variants, in all centers (including those that are not specialized in SM) will shorten the long diagnostic delay for this category of SM patients, which is currently around 7 years.
Conflict-of-interest disclosure: M.A. declares honoraria: Blueprint Medicines; consulting or advisory role: AB Science, Thermo Fisher. J.R. declares honoraria, consulting, and advisory role: Blueprint Medicines.