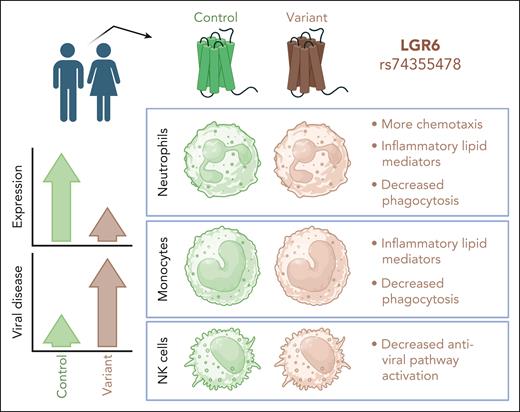
In this issue of Blood, Gomez et al1 find that loss of leucine-rich repeat-containing G protein-coupled receptor 6 (LGR6), a receptor for the proresolving mediator maresin 1 (MaR1), corresponds with altered activities of myeloid cells and a reduced antiviral response from natural killer (NK) cells. In the population, this deficiency positions patients to be more susceptible to viral disease (see figure).
The rs74355478 variant of the G protein-coupled receptor LGR6 reduces receptor expression on human neutrophils, monocytes, and NK cells. This is associated with variations in cellular phenotype and function across the 3 cell types, when studied ex vivo. On a population level, the variant appears to associate with increased risk for viral disease. Figure created with BioRender.com.
The rs74355478 variant of the G protein-coupled receptor LGR6 reduces receptor expression on human neutrophils, monocytes, and NK cells. This is associated with variations in cellular phenotype and function across the 3 cell types, when studied ex vivo. On a population level, the variant appears to associate with increased risk for viral disease. Figure created with BioRender.com.
Inflammation, injury, and repair occur in a continuum of phases, beginning with an inflammatory response and followed by antiinflammatory repair mechanisms. Neutrophils, monocytes, macrophages, and other immune cells likely contribute to not just the first phase, but also the transition to the second phase, and the resolvins have been implicated in the latter. These serve to regulate the magnitude of an inflammatory response and to promote its resolution when inflammation is no longer necessary. Our understanding of the various pathways that favor the regulation and resolution of inflammation are far from complete, and this is an active and exciting area of research.
The G protein-coupled receptor LGR6 is the receptor for proresolving mediator MaR1.2 MaR1 has been studied for its ability to limit inflammation in various ways, including inhibition of cytokine release from innate lymphoid cells, induction of regulatory T cells (Tregs), and potentiation of Treg function.3,4 Thus, LGR6 is positioned as a potentially critical molecule in limiting and resolving inflammatory responses. Yet a full picture of this receptor’s importance in cellular biology, and more broadly in disease, was not clear to date.
Variations in the gene for LGR6 can result in altered splice variants, some of which may influence receptor expression on the cell surface. In this study, Gomez et al examined a specific variant, rs74355478, found in the human population. Using samples from volunteers with and without this variant, they found that LGR6 expression was decreased on variant-positive neutrophils, monocytes, and NK cells, but not on monocyte-derived macrophages or CD8+ T cells. Variant neutrophils and monocytes harbored a more inflammatory profile of lipid mediators compared with controls. This was associated with reduction in ALOX12, an enzyme that catalyzes polyunsaturated fatty acids to generate lipid mediators including proresolving lipoxins.5 Neutrophils and monocytes were less phagocytic in the variant group, and neutrophils were more prone to traffic toward a chemotactic stimulus.
Based on these findings, the authors interrogated whether this variant of LGR6 might correlate with specific diseases in the population. Using available biobank data, their analysis suggested a substantially enriched incidence of viral disease in subjects with this variant and weaker enrichment for other conditions including pseudomonal pneumonia, migraine, cardiac dysrhythmia, uveitis, and insomnia. Additional studies of blood samples supported that a defective NK-cell response could underly the increase in viral disease incidence: variant-positive NK cells were hyporesponsive to agonists of multiple toll like receptors (TLRs) involved in antiviral responses (TLR3, 9, and 7/8).
Although neutrophils from the variant group appeared to be more inflammatory, they were deficient in phagocytosis, a key antimicrobial neutrophil function. This may be relevant for the moderate enrichment of pseudomonal pneumonia seen in the LGR6-variant population. Whether the altered phenotypes of LGR6-variant neutrophils are important for the elevated incidence of viral disease is not clear. Neutrophils are critically important for defense against bacterial and fungal pathogens, and they have been highlighted as key players in wound healing, yet their role in antiviral responses is less straightforward.6,7 Neutrophils may contribute to antiviral immunity through the production of various cytokines that can shape the immune milieu, and this may be deficient in the variant setting. LGR6-variant neutrophils produced less interleukin-12, interferon-γ (IFN-γ), and tumor necrosis factor-α (TNFα) in response to TLR agonists,1 which indicates a diminished T helper 1 cytokine profile, the cocktail that favors effective antiviral immunity. Thus, variant neutrophils may be inflammatory yet ineffectual, harboring inflammatory lipid mediators and trafficking with gusto, yet unable to contribute to robust antiviral immunity or pathogen clearance.
Beyond regulating inflammation, the role of variant neutrophils in wound healing and repair should be further investigated. Indeed, excessive recruitment plus a deficiency in resolving mediators could hamper a neutrophil’s pro-repair functions. Another area worth considering is whether presence of this LGR6 variant impacts putative neutrophil subsets, as some proposed subsets may be more antimicrobial and others more repair-like. Further refined analyses of neutrophil heterogeneity in LGR6-variant individuals could inform whether neutrophil states and/or polarity are affected, with implications for contexts such as cancer and autoimmunity, where neutrophil state is key.
The switch from classic monocytes to more alternative, repair-like monocytes was not directly investigated in this study, but it is plausible that this transition could be affected by lack of LGR6 expression. Indeed, intermediate monocytes, a transitional state between classic and nonclassic monocytes,8 were notably deficient in LGR6 in the variant group. Whether nonclassic monocytes are able to perform pro-reparative functions in this setting should be further investigated. Interestingly, it has been suggested that nonclassic monocytes may be specifically beneficial during viral challenge.9 Thus, a potentially dysfunctional nonclassic monocyte could have bearing on the population-level enrichment in viral disease.
The evidence that individuals with LGR6 variant are at increased risk for viral disease is most likely linked to changes in NK cells found in this setting, as NK cells are known to play important roles in antiviral defense.10 Their attenuated response to agonists of antiviral pathways (reduced CXCL10, TNFα, and IFN-β production) highlights this possibility. It thus may be that defective antiviral NK-cell responses, without proper proresolving mediators from myeloid cells, result in increased viral load and poor disease tolerance, engendering excessive inflammation and more severe clinical outcomes.
This study’s findings highlight the complex and cell-specific actions of the receptor LGR6. The existence of an LGR6-variant human cohort enabled study of this receptor in a real-world setting. Although the volunteer cohort is small, the data are provocative and implicate a single receptor in the coordinated balance between pro- and antiinflammatory pathways, beyond the proresolving functions of the receptor, expanding to the intrinsic antiviral response of NK cells. Whether these effects are all dependent on the actions of the ligand MaR1 is not clear. Nonetheless, this study sets the stage for understanding how LGR6 may serve as a linchpin in antiviral, and perhaps more broadly antimicrobial and inflammatory, host responses, coordinating cellular players of both lymphocytic and myeloid origin.
Conflict-of-interest disclosure: The authors declare no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal