In this issue of Blood, Chou et al report on D+ patients with sickle cell disease (SCD) who developed an anti-D and were successfully transfused with genotyped RHD+ red blood cells (RBCs).1
For over 30 years, it has been recognized that patients with SCD have a much higher risk of developing RBC antibodies after a transfusion than the general population. Thus, these patients are at risk of experiencing severe, even life-threatening, delayed hemolytic transfusion reactions.2,3 In 1990, Vichinsky et al first proposed that red-cell alloimmunization in patients with SCD could be partly explained by the differences between White donors and Black recipients in red-cell phenotype.4 To decrease this risk, in addition to ABO-compatibility testing, prophylactic antigen matching for Rh (C, E or C/c, E/e) and K antigens over ABO/RhD matching alone has been advocated for patients with SCD and is part of the American Society of Hematology 2020 guidelines for SCD transfusion support.5 In addition to Rh and K prophylactic matching, transfusing blood collected from Black donors was also put forward as a potential avenue for decreasing the risk of developing RBC antibodies. However, Rh antibodies against D, C/c, and E/e antigens remained prevalent despite antigen matching due to the presence of RH variants in both patients with SCD and Black donors.6
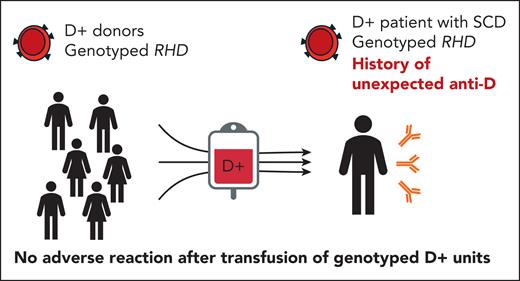
Chou et al describe a study of 5 patients with SCD and a conventional RhD who developed an unexpected anti-D after receiving between 42 and 341 of D+ units. It was suspected that the patients reacted to a potential RhD variant expressed on donor cells. When such a situation occurs, the current practice is to transfuse D− units to avoid a possible delayed hemolytic transfusion reaction, as these anti-D are considered potentially hemolytic. However, considering that patients with SCD, who are on a long-term red-cell exchange program, will require hundreds or even thousands of RBC units over the course of a lifetime, this puts considerable pressure on Rh− blood supplies, which are always fragile.
Furthermore, for patients who are heavily alloimmunized, or who have rare blood, relying strictly on Rh− blood donors limits considerably the probability of finding compatible blood donors. This is also true for D+ patients with RHCE variants or rare phenotype combinations, for whom the pool of compatible donors is limited.7 Thus, Chou et al showing that patients with SCD with conventional RhD and unexpected anti-D can be safely transfused with genotyped D+ blood is a game changer in transfusion practice (see figure).
Five D+ patients with sickle cell disease and genotyped conventional RHD, with a history of anti-D, received D+ units. Selected donors were D+ and had a genotyped conventional RHD. Patients and donors were genetically matched for RHD. The patients were closely monitored after each transfusion and no anti-D restimulation or adverse effects were observed.
Five D+ patients with sickle cell disease and genotyped conventional RHD, with a history of anti-D, received D+ units. Selected donors were D+ and had a genotyped conventional RHD. Patients and donors were genetically matched for RHD. The patients were closely monitored after each transfusion and no anti-D restimulation or adverse effects were observed.
Although this finding is exciting, one must recognize that many logistical and financial challenges must be addressed before such an approach can be implemented widely. Although recommended in the most recent American Society of Hematology guidelines,5 RBC genotyping of patients with SCD is still not routinely performed or available as standard of care. Moreover, blood donor testing is still limited because not all blood centers offer comprehensive RBC genotyping at a sufficient volume to allow proper genetic matching. If genotyping is available, the RHD gene may not be analyzed, because donor qualification for RhD often relies on serological analysis only. Thus, the ability of blood centers to provide genotyped units in sufficient numbers and in a timely manner remains a challenge. The technology used, whether a single-nucleotide polymorphism–based array platform or second- or third-generation sequencing, will affect the throughput and coverage. It is crucial that the chosen technology minimizes the risk of missing RHD variants, which could impact the outcome of the transfusion.
Another challenge that must be addressed is the availability of specialized personnel for the execution of analysis and interpretation of the diverse genetic results generated, both at the blood center and the hospital level. A modern laboratory information system capable of managing genetic results will also be needed to facilitate the processing of genotypes and patient-donor matching. Finally, the cost associated with this strategy will directly influence implementation of this approach. However, as the costs of genetic analyses tend to decrease over time, especially for sequencing, the feasibility of genetic matching increases.8
Regardless of these challenges, transfusion of genotyped RhD+ units for D+ patients with SCD and an unexpected anti-D is a promising approach that needs to be considered and more reports applying this or similar approaches must be shared. The strategy described by Chou and colleagues could also be applied to patients with the “normal” RHCE genotype but with unexpected anti-RhCE antibodies. Genotype-matched RBC units will play a key role in reducing the burden on Rh− blood supplies and providing the best-matched unit to our patients, thereby avoiding alloimmunization and delayed hemolytic transfusion reaction.
Conflict-of-interest disclosure: The authors declare no competing financial interests.