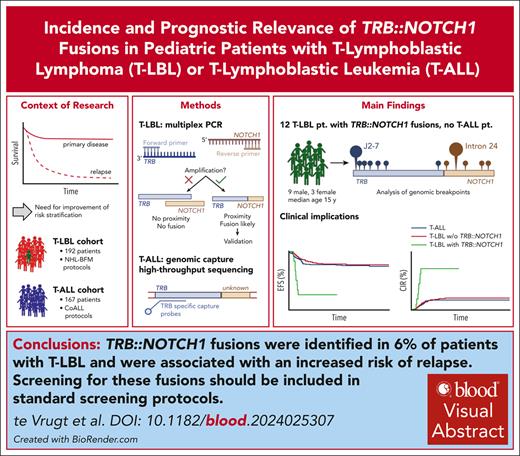
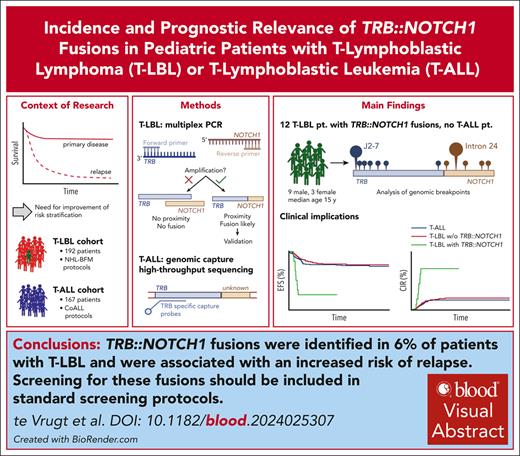
Key Points
This study reveals a novel recurrent genetic variant (TRB::NOTCH1) with significant prognostic relevance in T-LBL.
TRB::NOTCH1 is absent in the analyzed T-ALL cohort.
Visual Abstract
T-cell lymphoblastic lymphoma (T-LBL) and T-cell acute lymphoblastic leukemia (T-ALL) have common and distinguishing clinical and molecular features. Molecular prognostic factors are needed for T-LBL. We assessed the prevalence and prognostic impact of the T-cell receptor β (TRB)::NOTCH1 fusion in 192 pediatric patients with T-LBL and 167 pediatric patients with T-ALL, using novel multiplex polymerase chain reaction and genomic capture high-throughput sequencing techniques. The fusion was detected in 12 patients with T-LBL (6.3%) but in none of the patients with T-ALL (P = .0006, Fisher exact test). In T-LBL, the TRB::NOTCH1 fusion was associated with a significantly higher incidence of relapse (67% vs 17% in gene fusion-negative patients, P < .001, Fisher exact test). The breakpoint in TRB was most frequently located in J2-7 (n = 6). In NOTCH1, the breakpoints varied between exon 24 and 27. Consequently, a truncated NOTCH1 with its dimerization, regulation, and signal transduction domains gets controlled by strong TRB enhancer elements. This study reveals a novel recurrent genetic variant with significant prognostic relevance in T-LBL, which was absent in T-ALL. The TRB::NOTCH1 fusion in T-LBL suggests a possible unique pathogenic mechanism divergent from T-ALL. Further studies will validate the role of the TRB::NOTCH1 fusion as prognostic marker in T-LBL and elucidate its pathogenic mechanisms.
Introduction
T-cell lymphoblastic lymphoma (T-LBL) is the second most common subtype of non-Hodgkin lymphoma (NHL) in children and adolescents.1,2 Due to its characteristic clinical manifestation with a large mediastinal tumor, access to biological samples for molecular studies is limited. However, several groups contributed to the growing knowledge on the molecular profile of T-LBL.3-7 Acute T-cell lymphoblastic leukemia (T-ALL) is a more frequent but closely related disease that is well studied and shares clinical, morphologic, immunophenotypic, and genetic properties with T-LBL, feeding to ongoing discussion whether T-LBL and T-ALL represent 2 different entities or 2 variants in a spectrum of 1 disease.8,9 In regard to treatment and outcome, T-LBL and T-ALL are similar, and both share the urgent medical need because survival for patients who suffer a relapse is very poor.3,4 Over the past decade, there have been efforts to identify molecular markers of prognostic value for optimized risk group stratification.3,4,10 In T-LBL, NOTCH1 and FBXW7 variants in specific exons serve as favorable prognostic markers in the ongoing international trial LBL 2018 (NCT04043494). In a preliminary molecular analysis, we identified the chromosomal gene fusion t(7;9)(q34;q34.3) in selected T-LBL cases, resulting in the fusion of NOTCH1 with enhancer elements of the T-cell receptor β (TRB). This gene fusion has not been systematically investigated in T-LBL and has been rarely reported in T-ALL (<1%).11-15 This study was designed to investigate the incidence and prognostic relevance of TRB::NOTCH1 fusions in pediatric patients with T-LBL and T-ALL by employing novel sequence-specific multiplex polymerase chain reaction (mPCR) techniques and genomic capture high-throughput sequencing (gc-HTS).16
Study design
For T-ALL and T-LBL, clinical data of patient characteristics, diagnostics, treatment protocols, and outcomes are available from the clinical trial databases.
Among T-ALL cases registered at the German Cooperative Study Group for Childhood Acute Lymphoblastic Leukaemia (CoALL) Study Center and diagnosed between June 2005 and February 2022, a total of 167 pediatric and adolescent patients were eligible for the current study. Eligibility criteria are listed in the supplemental Data, available on the Blood website. Patients were treated uniformly according to CoALL 07-03 and 08-09, with informed consent obtained from their parents or guardians.17
T-LBL cases were identified at the NHL-Berlin-Frankfurt-Muenster (BFM) study center. All cases were documented after informed consent of patients, parents, or guardians (supplemental Table 1). These patients were diagnosed in Germany and received standard staging procedures and reference confirmation of diagnoses. All patients received treatment according to the NHL-BFM protocols for LBL,18 adhering to the established uniform treatment regimen. Paired initial and relapse samples were available for 11 T-LBL cases.
mPCR detection of TRB::NOTCH1 gene fusion in T-LBL cases
To detect the TRB::NOTCH1 genomic breakpoints, we established an mPCR method. This method was validated using genomic DNA from the SUP-T1 cell line (DSMZ-No. ACC 140) and samples from patients with T-LBL with known t(7;9)(q34;q34.3) gene fusion detected by gc-HTS, as well as the Jurkat cell line (DSMZ-No. ACC 282), and patient samples without gene fusion as negative controls. The mPCR was designed using a set of forward primers specific to all J and D segments of the TRB gene and a set of reverse primers targeting the NOTCH1 gene in a range from exon 24 to intron 27. This primer configuration is intended to amplify a product resulting from the proximity of TRB and NOTCH1 in a head-to-head configuration, indicative of the t(7;9)(q34;q34.3) gene fusion. To enhance the detection of head-to-head gene fusion events involving the J and D segments of the TRB gene and the NOTCH1 gene, ranging from exon 24 to intron 27, we employed a touchdown PCR protocol. All primers were tested both in a multiplex as well as in individual TRB- and NOTCH1-specific primer pair combinations with specific annealing conditions. The primer sequences, PCR setup, and cycling conditions are described in supplemental Tables 2 and 3.
High-throughput sequencing detection of TRB::NOTCH1 gene fusion in T-ALL and selected T-LBL cases
A genomic capture gc-HTS approach was applied for measurable residual disease marker analyses, which contained probes for TRB to identify all possible gene fusion partners (L. Behrmann, U. zur Stadt, and G. Escherich, unpublished data). We analyzed the genetic data from a subset of patients with T-ALL and a part of the patients with T-LBL to detect the TRB::NOTCH1 gene fusion.
Validation and precise determination of breakpoints via Sanger sequencing
All TRB::NOTCH1-specific PCR products generated were sequenced to determine the exact position of breakpoints. If the quality of the chromatograms was insufficient, PCRs were repeated with individual primer pairs of different combinations and the products were sequenced subsequently.
Statistical analysis
Event-free survival (EFS) was calculated from date of diagnosis to last follow-up or first event (relapse, secondary malignancy, or death of any cause). Probabilities were estimated using the Kaplan-Meier method with standard errors according to Greenwood and were compared with the log-rank test. Cumulative incidence functions for relapse (CIR) were constructed by the method of Kalbfleisch and Prentice and compared by using the Gray test.19 Differences in distribution of individual parameters among patient subsets were analyzed by the Fisher exact test.
Results and discussion
In total, 192 T-LBL and 167 T-ALL cases were screened for the TRB::NOTCH1 fusion gene via mPCR for all T-LBL and gc-HTS for selected T-LBL cases and gc-HTS for all T-ALL cases (supplemental Table 4). Our study reveals a previously unknown significant difference between T-LBL and T-ALL, detecting the TRB::NOTCH1 fusion in 12 out of 192 T-LBL cases (6%) but in none of the 167 T-ALL cases (P = .0006, the Fisher exact test). The gene fusion is significantly associated with patients’ age as it is absent in patients younger than 10 years of age (P = .0004) (Table 1).
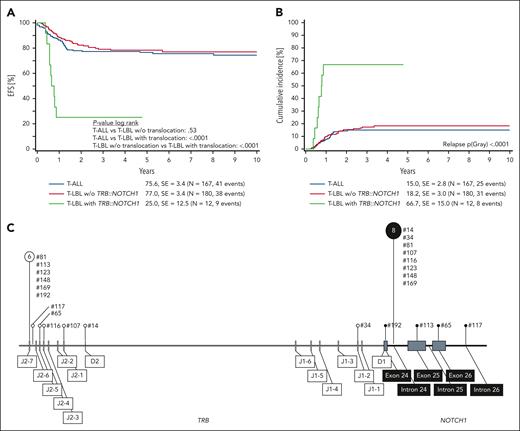
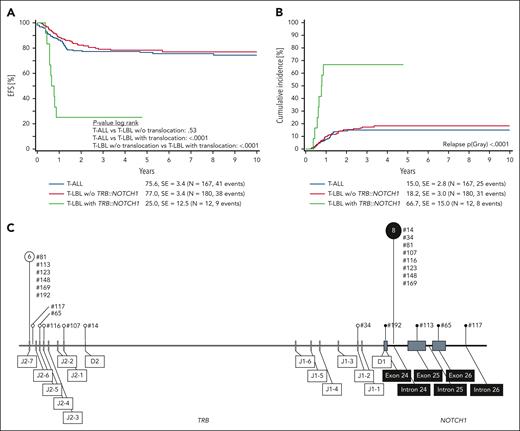
Comparing survival at 6 years from diagnosis, EFS is in the range of 75% to 80% and similar for T-ALL and T-LBL cases without TRB::NOTCH1 fusion, but EFS is significantly inferior with only 25% for T-LBL with TRB::NOTCH1 fusion (Figure 1A). This difference in EFS is attributed to increased CIR for T-LBL with TRB::NOTCH1 fusion of 67% at 6 years from diagnosis (Figure 1B). Outcome remained stable even if the analysis was focused on patients above 10 years of age (supplemental Figure 1).
Outcome according to TRB::NOTCH1 fusion status in pediatric patients with T-LBL and T-ALL. (A) Six-year EFS according to TRB::NOTCH1 status. (B) Six-year CIR. (C) Distribution of genomic breakpoints in TRB and NOTCH1 in pediatric T-LBL. Vertical lines represent the observed breakpoints along the TRB (left) and NOTCH1 (right) loci. The sizes of the pin circles correlate with the absolute number of breakpoints in our cohort. Numerical labels indicate event counts associated with specific breakpoints. Corresponding sample numbers are indicated next to the pins. SE, standard error; w/o, without.
Outcome according to TRB::NOTCH1 fusion status in pediatric patients with T-LBL and T-ALL. (A) Six-year EFS according to TRB::NOTCH1 status. (B) Six-year CIR. (C) Distribution of genomic breakpoints in TRB and NOTCH1 in pediatric T-LBL. Vertical lines represent the observed breakpoints along the TRB (left) and NOTCH1 (right) loci. The sizes of the pin circles correlate with the absolute number of breakpoints in our cohort. Numerical labels indicate event counts associated with specific breakpoints. Corresponding sample numbers are indicated next to the pins. SE, standard error; w/o, without.
Breakpoints within the TRB locus accumulated at the J2-7 and J2-6 segments, alongside pronounced clustering within intron 24 of NOTCH1. The nonrandom distribution of the breakpoints, specifically at positions 6 and 8, suggests a pattern of genomic instability events (Figure 1C and supplemental Tables 5 and 6).
The analysis of 11 T-LBL cases with available paired samples from initial diagnosis and first relapse identified the persistence of the TRB::NOTCH1 fusion at both time points in 4 cases and no case with newly occurring TRB::NOTCH1 gene fusion at relapse. Gc-HTS results of T-LBL samples suggest that the TRB::NOTCH1 fusion is most likely present in the vast majority of tumor cells. This reinforces the potential role of the gene fusion as pathogenic driver rather than an acquired genetic event.
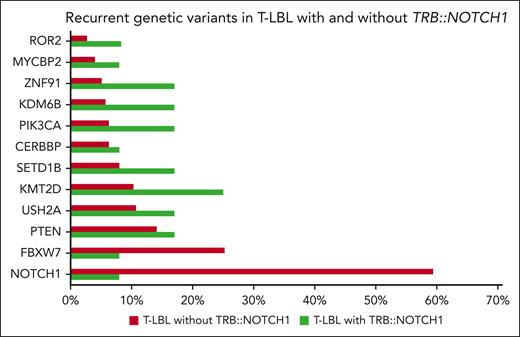
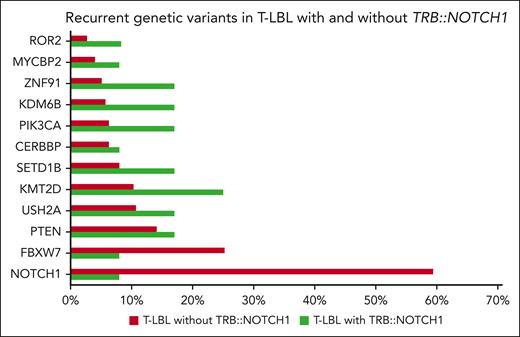
To learn more about the co-occurrence of other recurrent genetic variants described in T-LBL, we compared the 12 T-LBL cases with TRB::NOTCH1 fusion with the molecular profile of previously reported cases.3 We found a significantly lower rate of NOTCH1 mutations in those cases with TRB::NOTCH1 fusion (59% vs 8%, P < .001), although the incidence of other variants did not differ, potentially attributed to the small group of gene fusion-positive cases (Figure 2).
Recurrent genetic variants in pediatric T-LBL cases according to TRB::NOTCH1. Frequency of common genetic variants identified in T-LBL cases. Green bars denote the cohort of cases with the fusion, and red bars represent T-LBL without the fusion. Data were derived from previously analyzed patients on the molecular profile of T-LBL, including 80 genes, such as RAS, LEF1, PHF6, DNM2, and WT1. Despite these, no additional variants were detected in the 12 cases with TRB::NOTCH1 fusions.
Recurrent genetic variants in pediatric T-LBL cases according to TRB::NOTCH1. Frequency of common genetic variants identified in T-LBL cases. Green bars denote the cohort of cases with the fusion, and red bars represent T-LBL without the fusion. Data were derived from previously analyzed patients on the molecular profile of T-LBL, including 80 genes, such as RAS, LEF1, PHF6, DNM2, and WT1. Despite these, no additional variants were detected in the 12 cases with TRB::NOTCH1 fusions.
The ongoing clinical trial LBL 2018 (NCT04043494) integrates molecular genetic markers into the risk stratification process. The absence of variants in specific exons in NOTCH1 (exons 26, 27, and 34) and FBXW7 (exons 9, 10, and 12) delineates high-risk3,5,20 patients who are eligible for a randomized approach introducing an intensified experimental arm. Remarkably, except 1 case with a variant in FBXW7, no cases with TRB::NOTCH1 fusion show variants in the predefined exons of NOTCH1 and FBXW7, therefore stratify into the high-risk group. In the trial LBL 2018, central reference laboratories perform the molecular analysis of NOTCH1 and FBXW7. The context of the ongoing trial will provide evidence whether intensified treatment is sufficient to reduce CIR in this newly identified high-risk group of patients with T-LBL. Besides intensified standard chemotherapy, future treatment strategies for these patients at highest risk might necessitate new mechanisms of action like cellular immunotherapy or novel agents such as proteasome or BCL-2 inhibitors. International collaboration is needed to validate innovative treatment approaches and future risk group stratification including further evaluation of the role of minimal residual disease at end of induction and the potential need for allogeneic hematopoietic stem cell transplantation in first complete remission (supplemental Discussion).21 The mPCR detection of TRB::NOTCH1 gene fusion is valid, easy, and cheap and can be applied to all T-LBL cases recruited prospectively.
Yamamoto and colleagues noted that gene fusions at the 7q34 region involving NOTCH1 and TRB lead to an overexpressed truncated version of NOTCH1 under the regulatory control of TRB elements.12 Although gene fusions such as t(7;9) remain infrequent in the spectrum of T-ALL/LBL, our results offer substantial insights, particularly pinpointing the location of NOTCH1 breakpoints postexon 24, predominantly in intron 24 (supplemental Table 4). This specific breakpoint pattern is indicative of a ligand-independent mechanism activating the NOTCH1 pathway (supplemental Figure 2), which is considered a central driver in T-LBL pathogenesis and provides a potential target for therapeutic intervention.3,4
The incidence of NOTCH1 gene fusions in TRB in T-LBL, as opposed to their absence in T-ALL, may be molecularly attributed to discrepancies in DNA repair mechanisms and TRB rearrangement during T-cell development. Proteins central to the mismatch repair system and nonhomologous end-joining pathway, such as MLH1, MSH2, MSH6, and PMS2,22 are crucial in genomic stability.23,24 Deficiencies in TRB rearrangement or mismatch repair could facilitate chromosomal gene fusions between TRB and NOTCH1 and may influence the frequency and nature of genetic rearrangements between T-LBL and T-ALL, possibly contributing to the distinct pathogenesis observed in T-LBL.
In conclusion, we identified the TRB::NOTCH1 fusions in 6% of T-LBL but not in T-ALL. The occurrence of TRB::NOTCH1 was associated with an increased risk of relapse. This advocates for the inclusion of the TRB::NOTCH1 gene fusion by molecular methods in standard screening protocols. The gene fusion may serve as a robust biomarker for T-LBL prognostication and in formulating targeted therapeutic strategies.
Acknowledgments
This work is supported by grants from the Deutsche Kinderkrebsstiftung to the NHL-BFM Registry 2012 (DKS2014.11A/B) and grants from Medizinerkolleg Münster of University of Münster.
Authorship
Contribution: M.t.V. designed and performed research, contributed vital new analytical tools, collected data, analyzed and interpreted the data, and wrote the manuscript; J.W. performed research, collected data, analyzed and interpreted the data, and wrote the manuscript; G.R. performed research, contributed vital new analytical tools, collected data, analyzed and interpreted data, and wrote the manuscript; A.A. and S.M. performed research, collected data, and analyzed and interpreted data; K.S. and C.S. performed research and collected data; C.L.-K. performed research, collected data, analyzed and interpreted data, and wrote the manuscript; M.H. performed research and collected data; F.L. performed research, collected data, and wrote the manuscript; C.D.-W. and J.L. performed research and collected data; G.E. performed research, collected data, and analyzed and interpreted the data; U.z.S. and L.B. performed research, collected data, and analyzed and interpreted data; W.W. collected data and analyzed and interpreted the data; I.O. performed research, collected data, and analyzed and interpreted the data; M.Z. performed research, analyzed and interpreted data, and performed statistical analysis; M.M. performed research and analyzed and interpreted data; B.B. designed research, performed research, contributed vital new analytical tools, collected data, analyzed and interpreted data, wrote the manuscript, and supervised research; and all authors reviewed and approved the manuscript.
Conflict-of-interest disclosure: B.B. reports research funding by Roche and advisory role (Miltenyi, Novartis, AbbVie, Roche) with all activities on behalf of the institution and without any relevance for the current project. The other authors declare no competing financial interests.
Correspondence: Birgit Burkhardt, Department of Pediatric Hematology and Oncology, University Children’s Hospital Muenster, Albert-Schweitzer-Campus 1, 48149 Münster, Germany; email: birgit.burkhardt@ukmuenster.de.
References
Author notes
Data are available on request from the corresponding author, Birgit Burkhardt (birgit.burkhardt@ukmuenster.de).
Note on artificial intelligence (AI)-generated content: No AI-generated text or images were used in the preparation of the manuscript. Contributions of AI tools are limited to data acquisition and analysis, as specified in the graphics and legends.
The online version of this article contains a data supplement.
There is a Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.