In this issue of Blood, Merli et al1 present the largest pediatric cohort to date who received a T-cell receptor (TCR) αβ/CD19 cell-depleted human leukocyte antigen (HLA)-haploidentical transplant (haplotransplant) for acute leukemia, with outcomes as good as those reported in the T cell-replete HLA-matched setting. Not long ago, such outcomes were unthinkable.
The beginnings of haplotransplantation date back 40 years when novel methods to deplete bone marrow of T cells using soybean agglutinin and sheep red blood cells enabled the use of parental haplografts, thus saving the lives of children with severe combined immunodeficiency.2 Yet the log depletion of T cells necessary to control graft-versus-host disease (GVHD) prevented engraftment of haploidentical blood stem cells in immunocompetent hosts. This problem was overcome by the discovery that megadoses of stem cells could facilitate engraftment across HLA barriers.3 Haplotransplantation became a lifesaving alternative for patients with high-risk leukemia who lacked a suitable HLA-matched donor.4 However, posttransplant T-cell immune reconstitution expectedly lagged compared with T cell–replete grafts, increasing the risk of infection and relapse in haplotransplant recipients.
To improve the outcomes of haplotransplants, 2 different philosophies emerged. The first sought to simplify haplotransplantation by depleting proliferating alloreactive T cells in vivo using posttransplant Cytoxan. This humble yet effective strategy obviated the need for graft manipulation, making it accessible to centers worldwide. The second strategy focused on engineering increasingly sophisticated grafts by depleting CD3, CD3/CD19, and eventually TCR αβ/CD19 cells using magnetic beads. This approach selectively removed alloreactive GVHD-causing T cells along with B cells responsible for posttransplant lymphoproliferative disease while preserving valuable donor-derived immune cells, especially γδ T cells and natural killer (NK) cells that possess much needed anti-infectious and antitumor properties. The small numbers of residual TCR αβ T cells that escape the modern-day magnetic bead depletion may in fact be beneficial by contributing to engraftment and disease control. Importantly, this strategy intentionally omits pharmacologic posttransplant GVHD prophylaxis thus giving the graft the best chance at effective immune reconstitution. Across the globe, sizable cohorts of pediatric and young adult patients have confirmed that TCR αβ/B cell–depleted haplotransplantation is an attractive strategy to treat benign5 and malignant conditions6-8 in patients who lack an HLA-matched donor, with survival outcomes comparable to other donor sources,5,7 including HLA-identical siblings.6
Merli et al have built upon their previously published data6 and now report 213 pediatric patients treated with TCR αβ/CD19 cell–depleted haplotransplantation for acute leukemia at Italy’s largest pediatric center, with a median follow-up interval of almost 4 years. Their 5-year cumulative incidence of nonrelapse mortality (NRM) of 5.2% and cumulative incidence of relapse of 22.7% suggest that allogenic transplant outcomes are no longer defined by the availability of an HLA-identical sibling donor. Noteworthily, Merli et al found that the use of total body irradiation (TBI)-containing fully myeloablative conditioning (MAC) improved outcomes in children with acute lymphoblastic leukemia. These findings contrast with the results of a recently published US multicenter study (PTCTC ONC1401), which reports superior 2-year disease-free survival (DFS) and overall survival (OS) in children receiving reduced toxicity conditioning without TBI.7 This discrepancy may be the result of differential statistical power (152 patients in Italy vs 30 patients in the United States). Alternatively, significant discrepancies in NRM in patients receiving MAC/TBI in Italy (2-year DFS of ∼80%) vs the United States (2-year DFS of 60%) may point to more fundamental differences between the 2 patient cohorts or the administered treatment regimens. Surprisingly, and also in contrast to PTCTC ONC1401, Merli et al found that NK alloreactivity and donor KIR B-haplotype had no effect on survival. The authors speculate that the influence of NK alloreactivity may have been concealed by the impact of other effector cell populations, namely γδ T cells. Alternatively, one might wonder if even our most refined ways of phenotyping NK cells do not fully capture their activity in vivo. There is reason to trust that the sizable numbers of NK cells “of any color” exert beneficial anti-infectious and possibly antitumor effects; therefore, centers that lack advanced NK cell phenotyping capabilities may worry less about it.
Importantly, Merli et al found that patients with high minimal residual disease (MRD) who received more than 33 000 residual TCR αβ T cells per kilogram had a significantly increased DFS and OS without increase in GVHD. Does this observation point to precision strategies in which we envision tuning the number of T cells in the graft (safest as less GVHD-causing memory T cells) to attack the patient’s MRD?
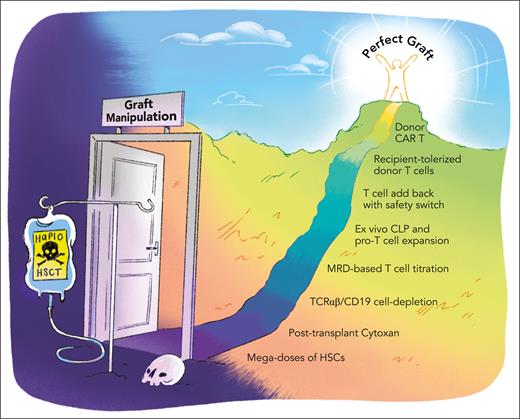
What is the next frontier in haplo? While we begin to regard GVHD and graft failure as problems largely belonging to the past, host immune reconstitution and long-term disease control remain the innate Achilles’ heel of the haplo approach. A myriad of strategies to safely add back T cells, for example, through transduction with molecular safeguards, have been trialed. Coculture of donor T cells with host antigen-presenting cells may hold promise in inducing tolerance while possibly conferring antileukemic effects.9 Recently, donor-derived chimeric antigen receptor (CAR) T cells for long-term disease control have been pioneered.10 Albeit compelling, all of these are costly approaches that yield single patient “bespoke” products. Are there any safe and economical strategies to enhance immune reconstitution on the horizon? For example, can we find ways to expand common lymphoid progenitors and pro-T cells to speed up immune reconstitution? The possibilities to build upon the TCR αβ/B cell–depleted haplo platform on our quest for the perfect graft seem limitless.
One would, however, be remiss not to point out that the extraordinary survival outcomes reported by Merli et al are the result of exceptional technical and clinical expertise acquired over years of iterative refinement. Translating these results into a resource-poor setting will almost certainly remain a challenge. As we inch closer to the “perfect graft” (see figure), high-income countries and the rest of the world drift further apart. Equity remains a frontier of a different kind. As such, we must appeal to those who preside over proprietary technology to consider equitable access to care-limiting resources. As much as the pediatric transplant community believes that access to care is a right and not a privilege, when it comes to engineering and receiving a near-perfect graft, a privilege it sure is.
Paving the way to the perfect graft. Scheme of different strategies that have been implemented or are being explored to optimize graft composition and improve outcomes after HLA-haploidentical allogeneic hematopoietic stem cell transplantation (HSCT). CLP, common lymphoid progenitor cell; HSC, hematopoietic stem cell. Professional illustration by Patrick Lane, ScEYEnce Studios.
Paving the way to the perfect graft. Scheme of different strategies that have been implemented or are being explored to optimize graft composition and improve outcomes after HLA-haploidentical allogeneic hematopoietic stem cell transplantation (HSCT). CLP, common lymphoid progenitor cell; HSC, hematopoietic stem cell. Professional illustration by Patrick Lane, ScEYEnce Studios.
Conflict-of-interest disclosure: K.G.W. is a scientific advisory board member of Garuda Therapeutics, Inc.