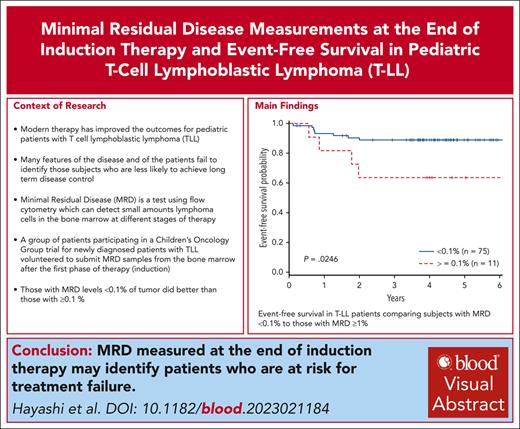
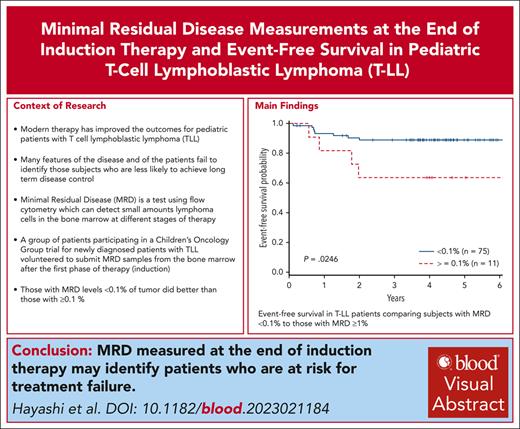
The level of MRD in the bone marrow at the end of induction correlates with event-free survival in T-cell lymphoblastic lymphoma.
MRD at the end of induction may be 1 of the few prognostic variables for event-free survival in pediatric T-cell lymphoblastic lymphoma.
Visual Abstract
Defining prognostic variables in T-lymphoblastic lymphoma (T-LL) remains a challenge. AALL1231 was a Children’s Oncology Group phase 3 clinical trial for newly diagnosed patients with T acute lymphoblastic leukemia or T-LL, randomizing children and young adults to a modified augmented Berlin-Frankfurt-Münster backbone to receive standard therapy (arm A) or with addition of bortezomib (arm B). Optional bone marrow samples to assess minimal residual disease (MRD) at the end of induction (EOI) were collected in T-LL analyzed to assess the correlation of MRD at the EOI to event-free survival (EFS). Eighty-six (41%) of the 209 patients with T-LL accrued to this trial submitted samples for MRD assessment. Patients with MRD <0.1% (n = 75) at EOI had a superior 4-year EFS vs those with MRD ≥0.1% (n = 11) (89.0% ± 4.4% vs 63.6% ± 17.2%; P = .025). Overall survival did not significantly differ between the 2 groups. Cox regression for EFS using arm A as a reference demonstrated that MRD EOI ≥0.1% was associated with a greater risk of inferior outcome (hazard ratio, 3.73; 95% confidence interval, 1.12-12.40; P = .032), which was independent of treatment arm assignment. Consideration to incorporate MRD at EOI into future trials will help establish its value in defining risk groups. CT# NCT02112916.
Medscape Continuing Medical Education online

In support of improving patient care, this activity has been planned and implemented by Medscape, LLC and the American Society of Hematology. Medscape, LLC is jointly accredited with commendation by the Accreditation Council for Continuing Medical Education (ACCME), the Accreditation Council for Pharmacy Education (ACPE), and the American Nurses Credentialing Center (ANCC), to provide continuing education for the healthcare team.
Medscape, LLC designates this Journal-based CME activity for a maximum of 1.0 AMA PRA Category 1 Credit(s)™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
Successful completion of this CME activity, which includes participation in the evaluation component, enables the participant to earn up to 1.0 MOC points in the American Board of Internal Medicine's (ABIM) Maintenance of Certification (MOC) program. Participants will earn MOC points equivalent to the amount of CME credits claimed for the activity. It is the CME activity provider's responsibility to submit participant completion information to ACCME for the purpose of granting ABIM MOC credit.
All other clinicians completing this activity will be issued a certificate of participation. To participate in this journal CME activity: (1) review the learning objectives; (2) study the education content; (3) take the post-test with a 75% minimum passing score and complete the evaluation at https://www.medscape.org/journal/blood; and (4) view/print certificate. For CME questions, see page 2111.
Disclosures
CME questions author Laurie Barclay, freelance writer and reviewer, Medscape, LLC, declares no competing financial interests.
Learning Objectives
Upon completion of this activity, participants will:
Describe clinical characteristics and outcomes of patients with T-cell lymphoblastic lymphoma (T-LL) enrolled in AALL1231, a Children’s Oncology Group phase 3 clinical trial for children and young adults (aged 1-30 years) newly diagnosed with T-cell acute lymphoblastic leukemia (T-ALL) or T-LL
Determine the association of minimal residual disease and other potential prognostic factors with event-free survival and other outcomes in patients with T-ALL or T-LL, based on findings of the AALL1231 trial
Identify clinical implications of outcomes and prognostic factors in T-ALL or T-LL, based on findings of the AALL1231 trial
Release date: May 16, 2024; Expiration date: May 16, 2025
Introduction
Traditional variables, such as stage or radiologic response to therapy, have failed to correlate with event-free survival (EFS) in recent trials in T-lymphoblastic lymphoma (T-LL).1-5 AALL1231 was a Children’s Oncology Group (COG) phase 3 clinical trial for newly diagnosed patients with T-cell acute lymphoblastic leukemia (ALL) or T-LL that randomized children and young adults (aged 1-30 years) to a modified COG-augmented Berlin-Frankfurt-Münster backbone to receive standard therapy (arm A) or with addition of bortezomib (arm B) during induction and delayed intensification (1.3 mg/m2 × 4 doses per block).6 We previously reported the favorable results of patients with T-LL receiving bortezomib.6 We now report our analysis of a subgroup of participants with T-LL who voluntarily submitted bone marrow samples at the end of induction (EOI) to assess the correlation of minimal residual disease (MRD) at EOI on EFS and overall survival (OS). Identification of variables that correlate with EFS is essential to develop risk-based therapies. MRD has shown to be a powerful prognostic tool for both B-cell ALL and T-cell ALL.7,8 Despite these advances in ALL, the relationship of EOI MRD to clinical risks in patients with T-LL is not known.
Study design
Newly diagnosed patients with T-LL, stage II to IV, were eligible for enrollment in COG ALL1231 (NCT02112916).6 Prior corticosteroid therapy was allowed if the administration was both <5 days within 7 days and <14 days in the 28 days before initiating induction therapy. Patients with T-LL were stratified as standard risk (SR) if they demonstrated <1% malignant cells in the bone marrow at diagnosis (minimally detectable disease [MDD]), had no central nervous system involvement, had no corticosteroid pretreatment, and demonstrated at least a partial response (PR) at the EOI. Intermediate risk (IR) patients had any of the following: corticosteroid pretreatment, >1% MDD, disease detectible in the central nervous system or testes at diagnosis, and still achieved at least a PR at the EOI. Very high-risk patients had any of the features of IR, but achieved no better than stable disease (SD) at the EOI. Bone marrow samples to assess MRD at the EOI were an optional submission for participants with T-LL, and these specimens were analyzed by flow cytometry, having previously demonstrated a validated sensitivity of 0.01% to assess its correlation to EFS.9,10
EFS was the primary outcome and defined as time from study enrollment to first event: death in induction or remission, refractory disease, relapse, second malignant neoplasm, or last contact date for those who were event free. OS was defined as time from study enrollment to death or last contact date. Proportions were compared using a χ2 test or Fisher exact test. Survival rates were estimated using the Kaplan-Meier method and standard errors.11,12 Multivariable analyses used Cox regression and included treatment arm and risk group. Per-protocol, subgroup analyses of overall outcomes, including by race, ethnicity, and sex, were performed. P < .05 was considered statistically significant for comparisons. Analyses were performed using SAS, version 9.4 (SAS Institute, Cary NC).
This study was conducted by COG under a National Cancer Institute–held Investigational New Drug application for bortezomib (NSC number 68129; Investigational New Drug number 58443). AALL1231 was approved by the Cancer Therapy and Evaluation Program, the Pediatric Central institutional review board, and participating center institutional review boards. Written informed consent and assent (if applicable) were obtained before study entry.
Results and discussion
AALL1231 accrued 209 patients with T-LL from 2014 to 2017 (supplemental Figure 1, available on the Blood website). At the EOI, 43.6% of patients were in radiologic remission, 55.4% had a PR, and 1% had SD or no response. There were 86 patients (41%) for whom EOI samples for MRD assessment were submitted. Demographic characteristics in this subgroup did not significantly differ from the cohort with T-LL (Table 1). There were differences observed in percentage blasts observed in the bone marrow at diagnoses (P < .0001) and stage (P = .0004), although stage was unknown for 55.7% of the patients who submitted a sample for MRD assessment. There was a history of corticosteroid pretreatment in 25.6% of patients; 62.2% had <1% of MDD in the bone marrow at diagnosis, resulting in 30.2% and 67.5% of patients assigned SR and IR, respectively. Those who participated in the MRD assessment had a higher representation of IR patients than those patients who did not (67.5% vs 38.2%; P = .0003). There were no very high-risk patients in this cohort, and 2.3% of patients could not be classified in a specific risk group. Complete response rate was 51.6%, 48.4% had a PR, and none had SD (Table 1).
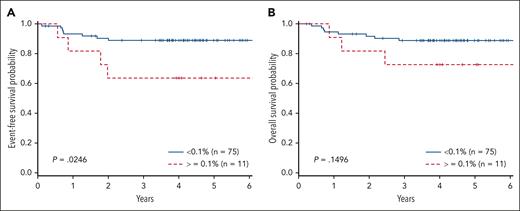
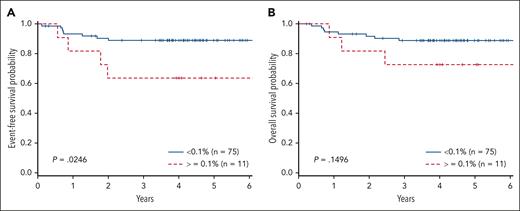
There was a significant difference in the 4-year EFS comparing arm A with arm B (78% + 8.1% vs 91.2% + 4.9%; P = .046). In addition, a significant difference was also observed in 4-year OS with those patients not receiving bortezomib (arm A, 78.8% + 8.1%) compared with those receiving bortezomib (arm B, 93.3% + 4.3%; P = .023), consistent with previously published results (Table 1). When examining MRD, there were 8 events in patients with MRD <0.1% (4 relapsed, 3 remission deaths, and 1 patient with progression) and 4 events in patients with MRD ≥ 0.1% (3 relapses, 1 remission death). Patients with MRD <0.1% (n = 75) at EOI had a superior EFS vs those with MRD ≥0.1% (n = 11) (89.0% ± 4.4% vs 63.6% ± 17.2%; P = .025). Analysis of the cohort above and below 0.01% failed to distinguish significant differences, possibly due to the small sample size (71 <0.01% vs 15 ≥0.01%). Furthermore, when examining the 4 patients with MRD <0.1 and >0.01, they are all free of disease. OS did not significantly differ between the 2 groups (88.9% ± 4.4% vs 72.7% ± 15.5%; P = .15) (Figure 1). IR and SR patients had similar EFS (arm A: 73.9% ± 7.5% vs 80.4% ± 6.7%; arm B: 87.2% ± 5.8% vs 90.5% ± 4.8%). Cox regression for EFS demonstrated inferior outcomes for those with MRD EOI ≥0.1% (hazard ratio [HR], 3.73; 95% confidence interval [CI], 1.12-12.40; P = .032), which was independent of treatment arm. OS failed to reach statistical significance for patients with MRD EOI ≥0.1% (HR, 2.714; 95% CI, 0.72-10.44; P = .14). Cox regression did not demonstrate a significant impact on EFS comparing arm A with arm B (HR, 0.57; 95% CI, 0.289-1.073; P = .080), or increasing MDD at diagnosis comparing <1% to 1% to 5% (HR, 0.830; 95% CI, 0.255-2.699) or >5% (HR, 2.67; 95% CI, 0.336-21.145; P = .141). Furthermore, MRD EOI ≥ 0.1% compared with EOI < 0.1% did not differ in complete response rates (55% vs 51%) or PR rates (45% vs 49%). In summary, MRD EOI was the only factor significantly associated with EFS.
MRD at EOI in T-LL. (A) EFS and (B) OS in patients with T-LL comparing MRD of <0.1% with MRD >1%.
MRD at EOI in T-LL. (A) EFS and (B) OS in patients with T-LL comparing MRD of <0.1% with MRD >1%.
Thus, in this phase 3 clinical trial, MRD <0.1% in the bone marrow at EOI for T-LL was associated with improved EFS, regardless of treatment arm for both univariate and multivariate analyses. These findings are consistent with a previous report examining MRD at the end of induction.13 Race, age, gender, risk group, MDD, and radiologic response to therapy were not prognostic. No chromosomal or molecular characterization of the disease was available. To our knowledge, this is the first report demonstrating that MRD at EOI is an independent risk factor correlating with EFS using a uniform means of assessing MRD. The findings most likely reflect a greater and more rapid reduction of disease burden, perhaps reflecting greater sensitivity to therapy, consistent with results from other pediatric lymphoma and leukemia trials. The study was limited as submission of EOI bone marrow specimens was voluntary and, thus, only 41% of the patients with T-LL enrolled had specimens available for MRD analysis. Larger numbers of patients would have permitted better analysis of MRD levels (0.01%-0.1%) and differences in treatment assignments due to risk stratification. Recent trials have failed to identify clear prognostic variables that would aid in risk stratifying patients for treatment.6,14 Given the paucity of available prognostic factors in this disease, incorporation of MRD at the EOI in large clinical trials will establish its value in risk stratification for future therapeutic trials to clarify the significance of this variable.
Acknowledgments
The authors thank the Cancer Therapeutic Evaluation Program for its support of AALL1231, the study's data safety monitoring committee, and the Pediatric Central Institutional Review Board. The authors especially acknowledge all participating Children’s Oncology Group investigators and the subjects and their families for participating in the study, without whom this report would not have been possible. Figures were generated using R version 2.13.1 (http://www.r-project.org).
This study was supported by National Institutes of Health (NIH), National Cancer Institute (NCI) grants U10CA180886 (M.L.L., D.T.T.), U10CA180899 (M.L.L.), U24CA196173 (M.L.L., D.T.T.), R01CA193776 (D.T.T., M.L.H., T.M.H., B.L.W., M.D.), and NIH, Eunice Kennedy Shriver National Institute of Child Health and Human Development grant X01HD100702 (D.T.T., M.L.L., S.P.H., M.D., S.S.W., K.P.D.), the Leukemia & Lymphoma Society (D.T.T.), NIH/NCI grant R03CA256550 (D.T.T., M.L.L., S.P.H., M.D., S.S.W., K.P.D.), St. Baldrick’s Foundation funding (M.L.L., S.P.H.), and the American Lebanese Syrian Association of Charities. M.L.L. is the Aldarra Foundation Endowed Chair, Seattle Children’s Research Institute, Seattle Children’s Hospital. S.P.H. is the Jeffrey E. Perelman Distinguished Chair in Pediatrics at The Children’s Hospital of Philadelphia. E.A.R. is a KiDS of NYU Foundation Professor at NYU Langone Health.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Authorship
Contribution: R.J.H., D.T.T., M.D., Z.C., M.L.H., P.H.-M., R.D.A., J.L.F., B.L.A., K.A., K.P.D., P.J.G., A.L., Y.H.M., S.P., E.S.S., S.L.V., S.S.W., P.A.Z.-M., C.M.B., M.L.L., S.P.H., and E.A.R. all contributed to the design of the study; D.T.T., A.L., S.S.W., and C.W. provided administrative support; D.T.T., M.D., B.L.W., Z.C., M.L.H., S.C., M.O., A.S., T.M.H., and R.R.M. participated in collection and assembly of data; and R.J.H., D.T.T., M.D., B.L.W., Z.C., M.L.H., K.A., K.P.D., J.L.F., P.H.-M., T.M.H., A.I.J., R.R.M., E.S.S., T.S., K.A.S., N.S., M.L.S., S.S.W., C.M.B., E.A.R., and C.E.A. performed data analysis and interpretation. All authors contributed to manuscript writing and final approval of manuscript.
Conflict-of-interest disclosure: S.P.H. received honoraria from Amgen; received consulting fees from Novartis; and owns common stock in Amgen. M.L.L. received consulting fees from MediSix Therapeutics. M.L.H. served on advisory boards for Novartis and Sobi. D.T.T. serves on advisory boards for Amgen, La Roche, Janssen, and Sobi. C.E.A. serves on advisory boards for Sobi and OPNA; and receives research support from Genentech. The remaining authors declare no competing financial interests.
Correspondence: Robert J. Hayashi, Division of Pediatric Hematology/Oncology, Department of Pediatrics, Washington University School of Medicine, 660 S Euclid Ave, St. Louis, MO 63110; email: hayashi_r@wustl.edu.
References
Author notes
The Children’s Oncology Group (COG) data sharing policy describes the release and use of COG individual subject data for use in research projects in accordance with National Clinical Trials Network (NCTN) Program and National Cancer Institute (NCI) Community Oncology Research Program (NCORP) guidelines. Only data expressly released from the oversight of the relevant COG data and safety monitoring committee are available to be shared. Data sharing will ordinarily be considered only after the primary study manuscript is accepted for publication. For phase 3 studies, individual-level deidentified datasets that would be sufficient to reproduce results provided in a publication containing the primary study analysis can be requested from the NCTN/NCORP Data Archive at https://nctn-data-archive.nci.nih.gov/. Data are available to researchers who wish to analyze the data in secondary studies to enhance the public health benefit of the original work and agree to the terms and conditions of use. For non–phase 3 studies, data are available following the primary publication. An individual-level deidentified dataset containing the variables analyzed in the primary results manuscript can be expected to be available on request. Requests for access to COG protocol research data should be sent to: datarequest@childrensoncologygroup.org. Data are available to researchers whose proposed analysis is found by COG to be feasible and of scientific merit and who agree to the terms and conditions of use. For all requests, no other study documents, including the protocol, will be made available and no end date exists for requests. In addition to above, release of data collected in a clinical trial conducted under a binding collaborative agreement between COG or the NCI Cancer Therapy Evaluation Program and a pharmaceutical/biotechnology company must comply with the data sharing terms of the binding collaborative/contractual agreement and must receive the proper approvals.
The online version of this article contains a data supplement.
There is a Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.