In this issue of Blood, El Otmani et al present new data advancing the concept that plasmin may play an important role in degradation of von Willebrand factor (VWF) within microvascular thrombi, and that a unique plasmin degradation product of VWF (cVWF) may serve as a biomarker of thrombotic thrombocytopenic purpura (TTP) and potentially other forms of microvascular thrombosis.1
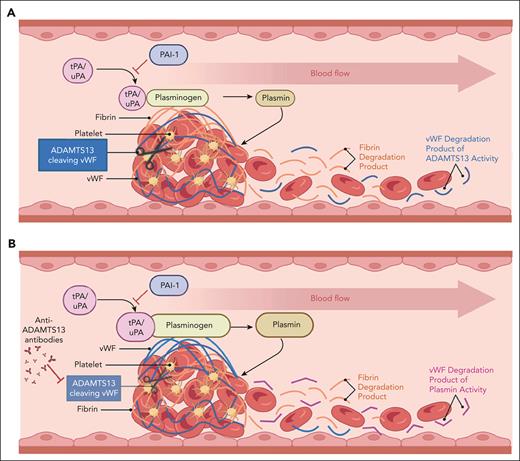
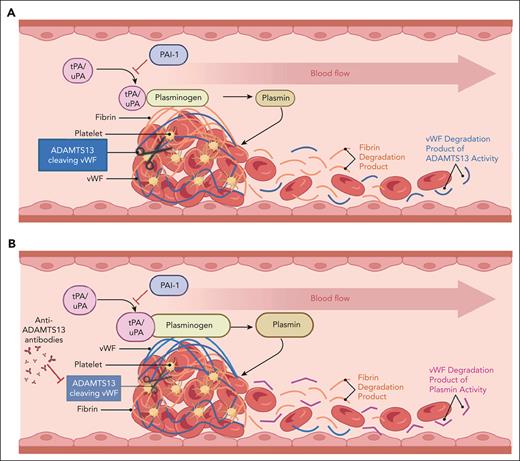
Endothelium lining the microvasculature is distinct from that lining larger vessels, and is engaged in dynamic regulation of inflammation, oxidative stress, hemostatic equilibrium, and other functions. Compared with thrombi involving larger vessels, microvascular thrombi occurring in disorders such as TTP are VWF- and platelet-rich, but relatively fibrin-poor, suggesting incorporation of largely unactivated platelets that do not promote thrombin generation and fibrin deposition (see figure).2 Moreover, plasmin has been shown in previous work to degrade platelet-VWF complexes on endothelial cells and in platelet-VWF agglutinates.3 In a murine model of TTP induced by ADAMTS13 antibodies, genetic deficiency of α2-antiplasmin accompanied by antibody-induced plasminogen activator inhibitor-1 neutralization ameliorated the microvascular thrombi that develop in this model.4 These findings, taken together with the increased levels of plasmin-antiplasmin complexes observed in patients with acute TTP, suggest that microvascular thrombi that occur in TTP may be degraded by plasmin, although sufficient plasmin for complete lysis is usually either not generated or rapidly inactivated. This observation has not yet been exploited therapeutically or diagnostically.
Macrovascular (A) vs microvascular (B) thrombosis. VWF (blue fibrils) is comparatively enriched in the microvascular thrombus, whereas fibrin (orange fibrils) is relatively enriched in the large-vessel thrombus. Products of thrombus degradation are depicted on the right (downstream blood flow) of each image. In the case of large-vessel thrombi, these consist mostly of fibrin degradation products (orange) and ADAMTS13 degradation products of VWF. In the case of microvascular thrombi, degradation products are enriched in plasmin-derived VWF degradation products (cVWF) relative to fibrin degradation products and VWF polypeptides derived from ADAMTS13 cleavage of VWF. Figure courtesy of Paresh Kulkarni.
Macrovascular (A) vs microvascular (B) thrombosis. VWF (blue fibrils) is comparatively enriched in the microvascular thrombus, whereas fibrin (orange fibrils) is relatively enriched in the large-vessel thrombus. Products of thrombus degradation are depicted on the right (downstream blood flow) of each image. In the case of large-vessel thrombi, these consist mostly of fibrin degradation products (orange) and ADAMTS13 degradation products of VWF. In the case of microvascular thrombi, degradation products are enriched in plasmin-derived VWF degradation products (cVWF) relative to fibrin degradation products and VWF polypeptides derived from ADAMTS13 cleavage of VWF. Figure courtesy of Paresh Kulkarni.
In their study, El Otmani et al prepared VWF-platelet agglutinates and demonstrated that they can be degraded by plasmin, releasing platelets back into suspension; during this process, plasmin generated unique VWF cleavage (cVWF) products. The authors then immunized Lama glama with human plasma VWF (Haemate P) and screened variable domain of heavy chain antibodies (VHH) clones against crude plasmin-cleaved VWF, with positive clones rescreened against plasmin-cleaved VWF subjected to a depletion step to remove intact VWF. VHH nanobodies consist of a single-chain, heavy-chain variable domain (VHH domain) of ∼15 kDa, which is derived from unique single heavy-chain antibodies expressed by camelids.5 Several cVWF-specific clones were generated and used to develop an antigen-capture enzyme-linked immunoassay (ELISA) in which plates were coated with one cVWF-specific capture VHH (G5), and bound cVWF detected with another cVWF-specific, biotinylated VHH (B9). This ELISA detected cVWF in buffer or citrate plasma with a detection limit of ∼2 ng/mL, but it did not detect intact VWF or ADAMTS13-generated VWF cleavage products. Moreover, no detectable cVWF was present in the plasma of normal individuals. By subsequently generating a series of VWF C-terminal truncation mutants, the authors demonstrated that the VWF A2 and A3 domains were required for cVWF epitope formation.
This novel cVWF ELISA was used to further explore the conditions under which cVWF was generated. cVWF was released from ristocetin-induced platelet and VWF-containing microthrombi generated in vitro, with up to 40% of the VWF within these microthrombi converted to cVWF within 30 minutes. The authors also demonstrated that plasmin degraded endothelial cell-derived, shear-unfolded VWF-platelet strings with ∼50% of the VWF converted to cVWF over 13.5 minutes.
After characterizing the capacity of plasmin for cVWF generation under these in vitro conditions, the authors compared circulating levels of cVWF in healthy controls with those of patients with active TTP or TTP in remission (although the median ADAMTS13 activity in this group was only 8.08%). Mean levels of cVWF were significantly increased in patients with active TTP compared with either of the other groups and correlated positively with those of plasmin-antiplasmin complexes and inversely with platelet counts. Unlike the in vitro situation, however, cVWF levels comprised only a small percentage of the total circulating VWF (∼0.1%). Finally, using a murine model of TTP induced by infusion of ADAMTS13−/− mice with recombinant VWF, infusion of Microlyse, a fusion protein consisting of a VHH targeting the CT/CK domain of VWF and urokinase plasminogen activator,6 caused a time-dependent accelerated formation of cVWF compared with saline-treated mice.
These findings demonstrate that plasmin is capable of lysing platelet and VWF-rich thrombin in the microvasculature, and that this process can be detected by measurement of circulating cVWF using a sensitive VHH-based ELISA. We now need to ask whether an assay such as this could have an impact on managing patients with TTP or other microvascular disorders, similar to that of the ADAMTS13 assay. We have learned in recent years that “remission” in TTP is a relative term, as patients with TTP may continue to develop silent brain infarcts even when in biochemical remission, with levels of ADAMTS13 above those believed to reflect active disease.7 Can increased levels of cVWF be of use in predicting which patients with previous TTP are at greatest risk of cerebral infarction and cognitive decline even with ADAMTS13 levels exceeding the 15% to 20% that predict TTP relapse8? In this regard, El Otmani et al showed that all 20 patients in TTP remission had low cVWF levels, but additional exploration of this may be warranted. A second observation of interest is that although the mean levels of cVWF were significantly higher in patients with active TTP, there was wide scatter in these levels, with most in the normal range. Does this suggest interindividual differences in microvascular plasmin-generating capacity? Do patients with low cVWF in the setting of active TTP have worse outcomes? Finally, extending this assay to other microvascular disorders, such as antiphospholipid antibody-induced microvascular disease,9 may provide additional pathophysiologic and diagnostic insight.
El Otmani et al should be congratulated for applying a VHH-based approach to developing a novel assay for measurement of plasmin-cleaved VWF and showing its potential utility in TTP. Future studies using this ELISA will likely expand our knowledge of the role of plasmin in microvascular thrombotic disease.
Conflict-of-interest disclosure: The authors declare no competing financial interests.