In this issue of Blood, Casey et al1 describe the representativeness of US lymphoma clinical trials that led to regulatory approvals over the past 10 years. By assessing the consistency of race/ethnicity, sex, and geographic region of participants in clinical trials with patients in disease registries, this study identifies inequities in clinical trial representation and highlights opportunities to improve the diversity of patients who enroll in practice-changing trials.2
Using 2 national US databases and the relevant primary articles reporting demographic information for randomized clinical trials that resulted in drug approvals for classic Hodgkin lymphoma (HL) and non-Hodgkin lymphoma (NHL) between 2011 and 2021, the distributions of race/ethnicity, sex, and other social and structural determinants of health were illustrated to identify areas of critical need for lymphoma trials and compared with the distribution of race/ethnicity and sex in cancer registries in the Surveillance, Epidemiology, and End Results Program.1 The authors report significant underrepresentation of Black, Hispanic/Latinx, and female patients in lymphoma clinical trials; and the gaps are striking. For example, nearly 1 in 6 patients diagnosed with NHL in the United States is Hispanic/Latinx; yet, Hispanic/Latinx patients accounted for only 1 in 19 patients in pivotal lymphoma clinical trials.
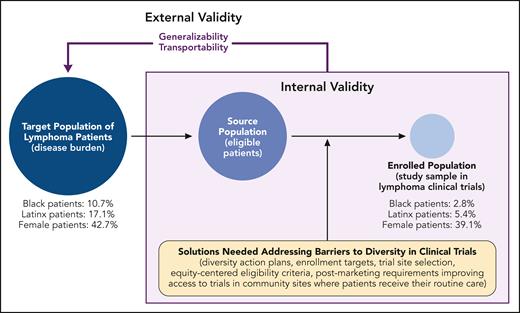
Herein, we apply a health equity lens (see figure) to lymphoma clinical trial participation by considering adverse social and structural determinants of health as barriers to accessing trials. Inequities in lymphoma outcomes by race/ethnicity, sex, HIV status, and sexual orientation are well documented,3 and they may originate, in part, from clinical trials for lifesaving cancer treatments lacking equitable representation. It will be important to better understand the reasons for this underrepresentation, which could relate to structural racism. Structural racism is racism that is normalized and legitimized by the policies and systems that govern society (eg, in housing, education, employment, health care, and criminal justice) and is maintained by, within, and across institutions.4 Within the context of clinical trial inequities, examples of structural racism may include lower access to trials in Black and Brown communities (ie, which clinical sites have certain trials open and available) and trial eligibility criteria that disproportionately exclude patients of color (eg, requirements for kidney function, which may disproportionately impact certain populations).5,6 It therefore may not be surprising to see the stark inequity in racial representation in NHL clinical trials (Black participants, 2.8%) relative to the burden of disease (Black patients, 10.7%) reported by Casey et al. Similar trends with inadequate demographic and geographic representation were described for classic HL in this study and in previous reports on pivotal clinical trials for leukemias and multiple myeloma.7 In an analysis of published trials that supported US Food and Drug Administration (FDA) approvals between 2008 and 2018, Loree et al8 reported similar findings across all hematology/oncology indications, where, in addition to inadequate representation of Black and Hispanic/Latinx patients, reporting of information on race/ethnicity overall and any subgroup analyses by race was infrequent and low. Greater representation of historically marginalized groups may reveal important factors related to the safety and efficacy of cancer treatments that affect all patients, and this underrepresentation undermines the external validity of the evidence driving our clinical and regulatory decision-making.
Graphical representation of internal and external validity of pivotal clinical trials for US lymphoma drug approvals between 2011 and 2021; example estimates are for NHL clinical trials reported by Casey et al. Professional illustration by Patrick Lane, ScEYEnce Studios.
Graphical representation of internal and external validity of pivotal clinical trials for US lymphoma drug approvals between 2011 and 2021; example estimates are for NHL clinical trials reported by Casey et al. Professional illustration by Patrick Lane, ScEYEnce Studios.
Readers should consider 2 aspects of external validity: generalizability and transportability.9 Issues with generalizability refer to concerns with making inference on the average treatment effect from a possibly biased sample of the target population back to the full target population. Transportability refers to making inference on the treatment effect for a target population when the study sample and target population do not overlap (partially or entirely). Poor representation of historically marginalized groups with lymphoma in clinical trials challenges both the transportability to populations of patients with lymphoma and the generalizability of the reported treatment effects in trials that inform regulatory approval.
Recognizing these challenges, in 2022, the US FDA released draft guidance on clinical trial diversity plans to improve enrollment of participants from historically excluded racial and ethnic populations in clinical trials.2 Legislation was subsequently passed requiring study sponsors to submit a diversity plan, which includes goals for study enrollment across racial/ethnic groups, specific steps to achieve them, and status updates of meeting those goals.10 The requirements might address the representativeness gap through a variety of ways. One way is through the standardized collection and reporting of data (eg, by following the Office of Management and Budget’s definitions of race and ethnicity). Casey et al noted that a considerable proportion of trials in the US databases and primary articles lacked demographic information entirely, including race/ethnicity and sex. Another way is the FDA’s encouragement to use various data sources, such as data from observational studies, to fill gaps in the drug development process. For example, the depth and breadth of these data can inform enrollment targets across diverse racial/ethnic populations (eg, diseases that are defined by the presence of rare biomarkers). Empirical data can also optimize eligibility criteria for specific populations of interest or inform where to open trials (ie, site selection); Casey et al report that counties with higher mortality rates and racial minority representation have low access to trials. In addition, pragmatic study elements and novel technologies that automate data capture may reduce site burdens associated with data collection and enable clinical trials in community sites that otherwise might not participate. Finally, if trial recruitment goals are not met, the draft guidance suggests that there may be postmarketing FDA requirements, and the data generated and collected during routine health care delivery (eg, electronic health records) can help inform safety and effectiveness in excluded populations.
In conclusion, Casey et al expand our understanding of inequities in the representation of historically marginalized groups in clinical trials that supported regulatory approval of treatments for classic HL and NHL. This timely report coincides with renewed efforts by lawmakers and regulators, requiring diversity plans to improve enrollment of participants from historically underrepresented racial/ethnic groups in clinical trials. We further highlight an opportunity for various data sources to improve diversity in clinical trials by informing and mitigating structural barriers to participation in the drug development process. Recently, the SWOG (Southwest Oncology Group) Cancer Research Network and Alliance for Clinical Trials in Oncology launched the Pragmatica-Lung Study (ClinicalTrials.gov identifier: NCT05633602), a phase 3 randomized trial for late-stage or recurrent non–small-cell lung cancer, with an innovative design mimicking conditions in clinical practice with eased eligibility criteria, simplified primary objectives, and limited data gathering requirements intended to speed accrual, ease administrative burdens, and expand patient eligibility and diversity. We are optimistic that some combination of increasing recognition of the gap in clinical trial representativeness, regulatory solutions, and innovations in trial design will bring us closer to a landscape where trials reflect the diversity of the end users: our patients.
Conflict-of-interest disclosure: G.S.C. and T.J.R. report employment with Flatiron Health (an independent member of the Roche group) and stock ownership in Roche.