In this issue of Blood, Nicolay et al demonstrate that targeting the NF-κB pathway with oral dimethyl fumarate (DMF) may be effective in certain patients with mycosis fungoides and Sézary syndrome (MF/SS).1 Building on their preclinical data demonstrating that DMF restores apoptosis sensitivity among malignant cells,2 the authors demonstrate the activity of single-agent DMF in a heterogeneous population of patients with relapsed or refractory stage IB-IV MF/SS in this phase 2 multicenter clinical trial.
Cutaneous T-cell lymphomas (CTCLs) often behave indolently in early stages in which treatment with topical steroids alone is sufficient, but approximately 25% will ultimately progress or present in advanced stage. In advanced stages, the disease can involve skin, lymph nodes, blood, and viscera. Unfortunately, few available therapies are able to target all disease compartments equally. Further complicating therapy, patients with MF/SS are at very high risk for invasive infections.3 Ubiquitous Staphylococcus aureus colonization and compromised immunity mean that any disruption in the skin barrier poses a risk of bacteremia, particularly among patients with erythroderma or widespread cutaneous involvement. Even peripheral administration of intravenous therapy can increase the risk of life-threatening sepsis. Oral therapies that do not significantly impair immunity are urgently needed for patients with advanced-stage disease. Currently, there are very few oral agents available for patients with CTCL. Vorinostat is the only oral agent approved in advanced stage, but its poor efficacy (global response ∼5%) and many adverse effects limit its use.2,4
DMF is a small-molecule compound currently approved for the treatment of multiple sclerosis and psoriasis. For psoriasis, DMF was approved based on a large phase 3 randomized clinical trial demonstrating improved clearance of psoriasis plaques compared with placebo.5 CTCLs are characterized by resistance to cellular death rather than active proliferation, making therapies that broadly target the cell cycle ineffective in many patients. Constitutive activation of the transcription factor NF-κB is a major mechanism of antiapoptosis among malignant CTCL cells and thus a prime therapeutic target.6 The authors’ preclinical work demonstrated that DMF inhibits NF-κB activity in CTCL cells while sparing healthy T cells, providing the rationale for this study (see figure).2
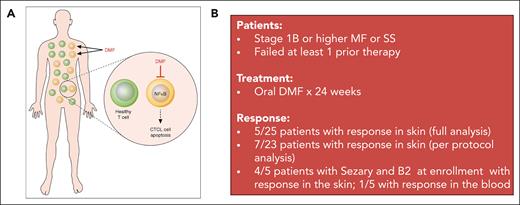
(A) Mechanism of DMF action in CTCL. DMF inhibits NF-κB activity in CTCL cells, inducing their apoptosis, while having minimal effect on healthy T cells. Professional illustration by Patrick Lane, ScEYEnce Studios; reproduced from Vancurova.11 (B) Clinical trial summary for the phase 2 study of DMF in CTCL.
(A) Mechanism of DMF action in CTCL. DMF inhibits NF-κB activity in CTCL cells, inducing their apoptosis, while having minimal effect on healthy T cells. Professional illustration by Patrick Lane, ScEYEnce Studios; reproduced from Vancurova.11 (B) Clinical trial summary for the phase 2 study of DMF in CTCL.
Grade 3 or higher events were rare among patients treated with DMF, and there were no significant (grade 3 or higher) hematologic toxicities reported on study. We can also glean some experience from DMF’s use in psoriasis; there, the most common adverse effects included gastrointestinal disturbances, whereas grade 3 leukopenia occurred in only ∼1% of patients.5 Current and prior studies indicate DMF targets the activated lymphocyte compartment only, having minimal impact on healthy T lymphocytes. In this trial, limited correlative studies provided further proof of principle: The single responder in the blood had higher baseline NF-κB activity in malignant T cells compared with 2 nonresponders.
Efficacy overall was moderate. Five of 25 patients in the full data analysis set (7 of 23 on the per-protocol set) had responses in the skin compartment (>50% reduction by modified Severity Weighted Assessment Tool); however, response in the blood was much lower, with only 1 patient achieving a response. These response rates are lower than more recently approved novel agents, brentuximab vedotin and mogamulizumab, which showed response rates in the skin of 77% and 42%, respectively, in phase 3 clinical trials.4,7 Unfortunately, DMF also did not have an appreciable effect on pruritis and quality of life. However, many of the patients on this study had low itch scores at baseline, making improvements difficult to measure. Durability is unclear at this time given the short interval follow-up and single time point for disease assessment (24 weeks). Notably, 11 patients discontinued the study prematurely, 7 of whom discontinued owing to disease progression or lack of efficacy.
A limitation of this and many CTCL trials is the lack of consistent baseline assessments and staging. Imaging was not routinely performed at baseline, nor were blood assessments such as flow cytometry or T-cell receptor gene rearrangement performed. Responses in lymph node and blood compartments, therefore, cannot be fully deduced. In practice, imaging assessments are often avoided in early-stage CTCL, despite data strongly demonstrating the inferiority of physical examination for staging compared with radiographic techniques.8 Some of this discrepancy is attributed to ambiguity of diagnosis in the early stage and the very indolent course of most patients with limited skin involvement. Added clarity to clinical and pathologic diagnostic criteria and consensus in defining the higher-risk groups in need of full staging procedures could rectify this problem.
It is likely that single-agent DMF may be insufficient, and future studies evaluating combinations with either skin-based approaches or systemic therapies would yield higher response rates. This study was small with a heterogenous population in terms of stage, limiting the power to identify subgroups that would derive the greatest benefit. The authors suggest that among patients with Sézary syndrome with B2 stage (absolute Sézary count 1000/μL) at the time of enrollment, the skin responses were higher, with 4 of 5 patients achieving a response in the skin. Nevertheless, the low response rate in the blood (1 of 5) and availability of more effective agents such as mogamulizumab, and soon the anti-KIR3DL2 antibody lacutamab,9 would limit its utility in Sézary syndrome as a single agent. Combinations with therapies like extracorporeal photopheresis, interferon, and/or mogamulizumab would be attractive options given their minimal impact on immunosuppression and should be investigated.
As the inter- and intraperson complexity of CTCL genomics is better defined, it has become increasingly clear that targeting a single protein or pathway is unlikely to lead to durable responses in the majority of patients.10 To improve outcomes in CTCL, we need a more rapid mechanism for drug approval evaluating multiple agents and combinations simultaneously to identify the most effective option for each molecular and clinical subtype. Novel trial designs such as platform or basket studies would allow the flexibility to integrate multiple biology-informed therapies that change over time with new drug approvals. To realize these opportunities, we need CTCL incorporated into more early-phase clinical trials. DMF is among the more promising agents for CTCL, but its target population and combination have yet to be identified.
Conflict-of-interest disclosure: P.B.A. is on the advisory boards of Kyowa Kirin and Daiichi Sankyo.