In this issue of Blood, Courtois et al identify interleukin-7 receptor (IL-7R) expression as a potential biomarker for predicting sensitivity to JAK pathway inhibition in T-cell acute lymphoblastic leukemia (T-ALL).1
To develop better directed treatment of T-ALL, Courtois et al divided patients into 3 main groups: those who express IL-7R (“expressers”), those who do not express IL-7R (“nonexpressers”), and those harboring IL-7R pathway–associated mutations (“mutants”). Using a panel of 46 patient-derived xenografts (PDX), the authors demonstrate ex vivo sensitivity to the JAK inhibitor ruxolitinib in IL-7R-expressing and -mutant patient groups, a finding which extends the potential clinical utility of ruxolitinib to the 70% of adult and pediatric patients with IL-7R-expressing T-ALL. As expected, ruxolitinib had no impact on IL-7R “nonexpresser” T-ALL cells. Using a multiomic approach, they show that “nonexpressers” have minimal IL-7R transcription owing to increased DNA methylation and minimal H3K4me3 signal (reflecting epigenetic downregulation of gene expression). In contrast, IL-7R-expressing T-ALL showed minimal methylation, high H3K4me3 signal, and high IL-7R transcription. They observed an inverse relationship between the ruxolitinib half maximal inhibitory concentration and H3K4me3 signal, suggesting that epigenetic modulation contributes to the greater vulnerability to ruxolitinib observed among IL-7R-expressing T-ALL. The authors also show that IL-7R-mutant T-ALL demonstrates strong functional dependency on BCL2 and, thus, ex vivo venetoclax sensitivity, whereas IL-7R-expressing T-ALL can be “primed” by ruxolitinib to enhance functional BCL2 dependency and, in turn, responsiveness to venetoclax. To demonstrate the therapeutic feasibility of JAK1/BCL2 dual inhibition, Courtois et al present 2 patients with relapsed/refractory T-ALL who both achieved transient responses with cotargeted therapy.
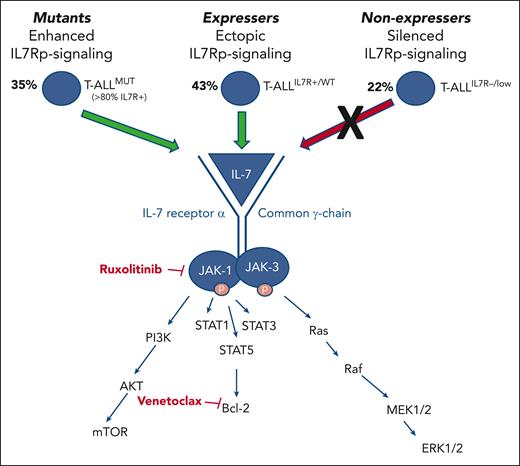
The supporting role of IL-7R signaling in T-ALL is well established. In 2011, Silva et al demonstrated that IL-7, a cytokine and hematopoietic growth factor critical for T lymphoid development, can promote T-ALL progression,2 and Zenatti et al identified somatic gain-of-function mutations associated with the IL-7R pathway (IL-7Rp) in 9% of patients.3 Because of the inherent relationship between IL-7/IL-7R signaling activation and multiple downstream pathways, namely, JAK1/2, BCL2, and PI3K/AKT/mTOR, these foundational data cultivated an interest in targetable IL-7/IL-7R–associated pathways as potential therapeutic vulnerabilities in T-ALL (see figure). The ensuing literature demonstrated the role of cooperating mutations with IL-7R mutations in promoting leukemogenesis4 and showed that high IL-7R expression corresponded to IL-7R-mutant gene expression signatures, begetting sensitivity to targeted inhibition of JAK, STAT, PIM, and PI3K/Akt signaling.5 These data, highlighting a central role for the IL-7R pathway, provided a new target for therapies in T-ALL. Preclinical models of JAK inhibition have since primarily focused on early T cell precursor (ETP) T-ALL, a particularly aggressive T-ALL subtype with unique immunophenotypic and genomic features thought to be dependent on JAK/STAT signaling. Maude et al demonstrated aberrant JAK/STAT activation and IL-7 responsiveness in ETP T-ALL and showed preclinical efficacy of ruxolitinib in producing an in vivo leukemia response in a PDX model.6
Depiction of 3 distinct categories of patients with T-ALL. The 3 categories include those with IL-7Rp mutations with constitutive pathway activation (“mutants”), those with IL-7R expression with ectopic IL-7Rp activation (“expressers”), and those with silenced IL-7Rp signaling (“nonexpressers”). “Mutant” and “expresser” groups constitute approximately 70% of patients with T-ALL and are characterized by activation of JAK/STAT signaling and reinforced BCL2 activation. Patients in these groups are predicted to be sensitive to the JAK inhibitor ruxolitinib and the BCL2 inhibitor venetoclax.
Depiction of 3 distinct categories of patients with T-ALL. The 3 categories include those with IL-7Rp mutations with constitutive pathway activation (“mutants”), those with IL-7R expression with ectopic IL-7Rp activation (“expressers”), and those with silenced IL-7Rp signaling (“nonexpressers”). “Mutant” and “expresser” groups constitute approximately 70% of patients with T-ALL and are characterized by activation of JAK/STAT signaling and reinforced BCL2 activation. Patients in these groups are predicted to be sensitive to the JAK inhibitor ruxolitinib and the BCL2 inhibitor venetoclax.
Courtois et al add substantially to this literature by demonstrating the potential utility of IL-7R expression as a biomarker to predict ruxolitinib sensitivity in up to 70% of patients with T-ALL. Although the value of dual JAK and BCL2 inhibition in the setting of JAK pathway–activating mutations has been previously suggested,7 Courtois et al expand upon this concept by demonstrating consistent synergy using 10 mutant T-ALL samples with a range of IL-7R-pathway mutations in a PDX model. The authors also found that ruxolitinib priming enhanced venetoclax responsiveness in nonmutant IL-7R-expressing T-ALL in a PDX model. These data reveal an opportunity to apply IL-7R-expression status to predicting which patients might most benefit from IL-7/IL-7R pathway–associated targeted inhibition in the clinical setting, either as an adjunct to up-front T-ALL therapy in the context of future trials or as a salvage modality for bridging to stem cell transplant.
An important future consideration will involve the optimal therapeutic time point in which to incorporate ruxolitinib and venetoclax. Delgado-Martin et al demonstrated that glucocorticoid resistance (GC) represents a particular vulnerability and predictor of relapse in ETP and some non-ETP T-ALL and that removal of IL-7 or JAK inhibition resensitized GC-resistant, IL-7–dependent T-ALL to GC.8 Mechanistically, GC exposure directly causes upregulation of IL-7R expression, leading to downstream BCL2 upregulation, which in turn promotes leukemia survival. However, targeted inhibition of the IL-7R/JAK/STAT5/BCL2 signaling axis could reverse this phenomenon, recover GC sensitivity, and synergistically provide therapeutic benefit in T-ALL.9 These data suggest that dual inhibition combined with GC-rich treatment phases could potentially abrogate GC resistance in select cohorts. However, as in any aggressive and heterogeneous cancer, single-cell data have already introduced the concern that select T-ALL cell populations with variable responsiveness to inhibition may persist at relapse.10 Thus, the field will certainly benefit from efforts to combine synergistic IL-7R pathway–targeted therapies in a multifaceted attempt to avoid therapeutic escape, as here introduced by Courtois et al.
Conflict-of-interest disclosure: The authors declare no competing financial interests.