In this issue of Blood, Xiao et al1 describe, for the first time, a role for bone morphogenetic protein 5 (BMP5) in the control of hepcidin, the master regulator of systemic iron homeostasis.
Hepcidin is a peptide hormone produced by the liver, which limits iron absorption from the diet and iron release from stores. Hepcidin acts by occluding and degrading the sole iron exporter, ferroportin. Hepcidin is transcriptionally regulated by different pathways, among which a major role is played by the BMP suppressor of mothers against decapentaplegic (SMAD) system. On BMP’s binding, the BMP type II receptors (BMP receptor 2 and activin A receptor type 2A (ACVR2A)) phosphorylate type I receptors (activin receptor-like kinase 2 (ALK2) and activin receptor-like kinase 3 (ALK3)), which, in turn, phosphorylate SMAD1/5/8. These proteins bind the cargo SMAD4; then, the complex translocates into the nucleus to activate the transcription of genes carrying a BMP-responsive element, including hepcidin.2
Two BMP ligands mainly produced by liver sinusoidal endothelial cells (LSECs), BMP23,4 and BMP6,5,6 are relevant in hepcidin modulation. BMP2 maintains basal hepcidin levels binding ALK3, whereas BMP6, upregulated by liver iron, activates the pathway in conditions of tissue iron accumulation, preferentially via ALK2.2 Despite the crucial and nonredundant roles of these 2 ligands, iron is still able to activate the BMP-SMAD-hepcidin pathway in double LSEC Bmp2 knockout (KO) and global Bmp6 KO mice, suggesting that at least 1 additional ligand is involved in the upregulation of hepcidin. In the present study, Xiao et al demonstrate that BMP5 is likely the missing piece of the puzzle.
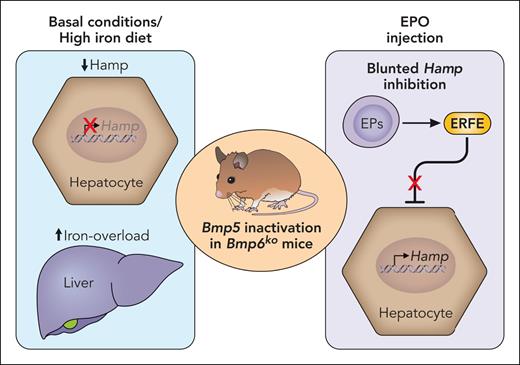
The role of BMP5 in the physiological regulation of hepcidin is marginal compared with that of BMP2 and BMP6. Indeed, mice lacking Bmp5 in the whole organism do not show dysregulation of the iron regulatory system, with the sole exception of a modest decrease in hepcidin levels when 10 days old. This is potentially reminiscent of a defect during fetal life, which is resolved in adulthood. However, when these animals are challenged with an iron-poor or a high-iron diet, they accumulate more iron in the liver and maintain inappropriately low hepcidin levels. This finding proves that BMP5 contributes to the transcriptional activation of hepcidin in response to both low and high iron, when Bmp6 is virtually absent or maximally induced. In agreement, Bmp5 inactivation in mice lacking Bmp6 (both globally or specifically in LSECs) dramatically reduces hepcidin levels, worsening both hepatic and extrahepatic iron overload, an effect exacerbated by feeding animals a high-iron diet (see figure). In Bmp6 total KO, the loss of Bmp5 causes an increased mortality, irrespective of the degree of iron overload, which is comparable in both Bmp6 global and LSEC-specific KO, but likely related to a redundant nonendothelial developmental role of BMP6 and BMP5, which remains to be elucidated.
The loss of Bmp5 severely impacts the phenotype of Bmp6-deficient (Bmp6ko) mice. In basal conditions and following a high-iron diet, Bmp5 deficiency further decreases hepcidin (Hamp) levels and worsens hepatic and extrahepatic iron overload in mice lacking Bmp6 both in the whole organism and selectively in LSECs. Conversely, following a erythropoietic stimulus, erythroferrone (ERFE) fails in downregulating hepcidin transcription in mice lacking both BMP5 and BMP6. EP, erythroid progenitor; EPO, erythropoietin. Professional illustration by Patrick Lane, ScEYEnce Studios.
The loss of Bmp5 severely impacts the phenotype of Bmp6-deficient (Bmp6ko) mice. In basal conditions and following a high-iron diet, Bmp5 deficiency further decreases hepcidin (Hamp) levels and worsens hepatic and extrahepatic iron overload in mice lacking Bmp6 both in the whole organism and selectively in LSECs. Conversely, following a erythropoietic stimulus, erythroferrone (ERFE) fails in downregulating hepcidin transcription in mice lacking both BMP5 and BMP6. EP, erythroid progenitor; EPO, erythropoietin. Professional illustration by Patrick Lane, ScEYEnce Studios.
At difference with BMP2 and BMP6, which are mainly produced by LSECs in response to iron levels, Bmp5 is not preferentially expressed in any liver cells and is not transcriptionally activated by iron. However, its activity appears strongly dependent on iron availability, raising the possibility that the metal controls BMP5 at the posttranscriptional level. However, the lack of specific antibodies currently precludes the possibility of addressing this point. Also, how BMP5 works, which receptors it uses, whether it can form heterodimers with BMP2, BMP6, and/or other BMPs, its potential involvement in the fetal iron homeostasis, and why BMP6 levels are limiting for the function of BMP5 are still unsolved issues, which likely will be the focus of further studies from the same and other groups in the field.
BMPs are crucial not only for the iron-mediated regulation of hepcidin, but also for hepcidin control by the expanded erythropoiesis. In response to erythropoietin (EPO) stimulation, developing erythroblasts produce the erythroid regulator erythroferrone (ERFE), which inhibits hepcidin transcription to increase iron supply needed for hemoglobin production.7 ERFE functions by binding and sequestering BMPs, mainly BMP6, but also BMP5 and BMP7.8 However, EPO injection is still able to suppress hepcidin in Bmp6 global KO,9 proving that other BMPs likely contribute to the ERFE-mediated hepcidin downregulation. The current study demonstrates that BMP5 is involved in this regulation. Indeed, EPO injection, although properly inhibiting hepcidin in Bmp5-deficient mice, is completely ineffective in male mice lacking both Bmp5 and Bmp6 (see figure). However, residual hepcidin suppression is observed in double-mutant female mice, which have higher basal hepcidin levels, raising the possibility that other BMPs might play a role.
Overall, this work reveals BMP5 as a novel player in the complex regulation of hepcidin and systemic iron homeostasis, and it paves the way for more comprehensive studies on the function of this, and potentially other, BMP ligand. From a translational perspective, interfering with their function might potentially become a novel therapeutic opportunity for iron and erythroid disorders due to excessive hepcidin production, in line with recent results obtained with anti-BMP6 agents.10
Conflict-of-interest disclosure: The author declares no competing financial interests.