Abstract
T-cell lymphoblastic lymphoma (T-LLy) and T-cell acute lymphoblastic leukemia (T-ALL) have historically been considered a spectrum of the same disease. However, recent evidence demonstrating differential responses to chemotherapy raise the possibility that T-LLy and T-ALL are distinct clinical and biologic entities. Here, we examine differences between the 2 diseases and use illustrative cases to highlight key recommendations on how to best treat patients with newly diagnosed and relapsed/refractory T-LLy. We discuss results of recent clinical trials incorporating use of nelarabine and bortezomib, choice of induction steroid, role of cranial radiotherapy, and risk stratification markers to identify patients at highest risk of relapse and to further refine current treatment strategies. Because prognosis for relapsed or refractory T-LLy patients is poor, we discuss ongoing investigations incorporating novel therapies, including immunotherapeutics, into upfront and salvage regimens and the role of hematopoietic stem cell transplantation.
Introduction
Lymphoblastic lymphoma (LLy) comprises 25% of pediatric non-Hodgkin lymphoma (NHL) cases. The majority (∼75%) are derived from early T-cell progenitors. The clinical distinction between T-cell acute lymphoblastic leukemia (T-ALL) and T-LLy relies upon the arbitrary cutoff of greater or less than 25% lymphoblast involvement in the bone marrow (BM), respectively. Currently, most cooperative groups consider T-LLy and T-ALL a spectrum of the same disease and treat using leukemia-based therapies.1-3 Similarities between T-ALL and T-LLy include malignant transformation of thymocytes that can infiltrate the mediastinum and other lymphoid organs, with or without involvement of peripheral blood, BM, and/or cerebrospinal fluid (CSF). There is significant overlap in presenting features because patients with T-ALL can present with lymphomatous disease and patients with T-LLy often have marrow involvement (5% to 25%). Moreover, approximately 40% of patients with relapsed T-LLy have evidence of isolated marrow disease.3,4 In addition, the frequency of early T-cell precursor phenotype is similar in patients with T-ALL or T-LLy.5
Despite these similarities, recent biologic and clinical data raise the possibility that T-LLy and T-ALL may represent distinct entities.6,7 Clinically, patients with ataxia telangiectasia are predisposed to T-ALL but almost never develop T-LLy.8 Immunophenotypic and gene expression studies demonstrate unique genetic driver mutations in T-LLy and higher expression of the cell–cell adhesion markers S1P1 and ICAM1 in T-LLy blasts.8-10 The latter may explain why T-LLy cells remain embedded in lymphoid tissues in proximity of stromal cells and less frequently involves the central nervous system (CNS) at diagnosis or relapse.8-10 Perhaps most importantly, recent data (discussed below) indicate that T-ALL and T-LL can have distinct responses to the same chemotherapy and may benefit from different therapeutic approaches.11
Relapsed T-ALL and T-LLy portend a poor prognosis with salvage rates of <25% and <15%, respectively, highlighting the importance of cure with front-line therapy. Ongoing research seeks to identify prognostic markers at diagnosis that can be used for early intensification of therapy to improve outcomes for high-risk patients. Currently, there are many unanswered questions on what the “standard-of-care” regimen should be and how patients should be risk stratified given the unclear prognostic significance of minimal disseminated disease at diagnosis (MDD), minimal residual disease (MRD) at end of induction (EOI) and end of consolidation (EOC), fluoro-deoxy-glucose (FDG) positron emission tomography (PET) responses, and the impact of genetic alterations. There is also no agreement on how to best treat patients with relapsed/refractory (r/r) disease. Using illustrative cases and informed by our experience with Children’s Oncology Group (COG) cooperative trials, this manuscript discusses current data regarding these gaps in our understanding and our approach to patients with primary and r/r T-LLy.
Patient 1: newly diagnosed stage 4 T-LLy
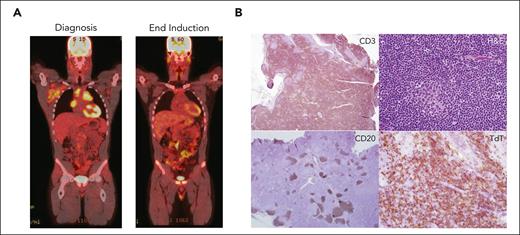
A previously healthy boy aged 16 years presented with cervical and supraclavicular adenopathy on physical examination and a mediastinal mass on chest x-ray. Complete blood count was normal. PET/computed tomography (PET/CT) imaging showed a large FDG-avid anterior mediastinal mass (Figure 1). Excisional biopsy of a supraclavicular lymph node revealed T-LLy (TdT+, CD2+, CD3+, CD5+, CD7+, myeloperoxidase negative, CD19−, CD34−). BM evaluation demonstrated 8% T lymphoblasts by flow cytometry. CSF was negative for lymphoblasts. Cytogenetics were noninformative and molecular profiling from a next-generation sequencing panel was positive for a NOTCH1 mutation. The patient was diagnosed with Stage 4 T-LLy. What is the optimal upfront therapy for this patient? Should prednisone or dexamethasone be used as the induction steroid? Should the treating oncologist use bortezomib and/or nelarabine? What is the optimal CNS-directed therapy? Are there prognostic biomarkers and imaging response that can be used for risk stratification and to guide therapy decisions?
Evaluation of a patient with newly diagnosed T-LLy. (A) PET-CT at diagnosis (left) and EOI (right). (B) Typical histologic and immunohistochemical findings in a patient with T-LLy. Small round blue cells are seen histologically. Immunohistochemical analysis reveals cytoplasmic CD3 and TDT positive, CD20 negative.
Evaluation of a patient with newly diagnosed T-LLy. (A) PET-CT at diagnosis (left) and EOI (right). (B) Typical histologic and immunohistochemical findings in a patient with T-LLy. Small round blue cells are seen histologically. Immunohistochemical analysis reveals cytoplasmic CD3 and TDT positive, CD20 negative.
Importance of early treatment intensification
Given the poor salvage rates for relapsed T-LLy, early intensification of therapy to improve outcomes is essential.3,12,13 Children with NHL, including those with T-LLy, were historically treated with nitrogen mustard derivatives and radiation therapy, with or without maintenance regimens of single chemotherapeutic agents.14 The need for early intensification to curtail early relapsed was exemplified by the superior outcome in patients with T-LLy treated on the NHL-BFM90 trial with a leukemia-like Berlin-Frankfurt-Munster (BFM) approach that added a delayed intensification (DI) phase for a total therapy duration of 24 months and eliminated local radiotherapy. This resulted in an estimated 5-year event free survival (EFS) of 90% (confidence interval, 0.82-1.0).2,15 These results led to the adoption of ALL therapy for patients with T-LLy, with patients with T-ALL and patients with T-LLy being treated on the same clinical trial in many cooperative groups.3,16,17
What is the optimal upfront therapy for T-LLy?
Both prednisone and dexamethasone have been used in induction (Table 1). Only 1 trial directly compared the 2 steroids. Thus, assessing the relative efficacy of each steroid by comparing outcomes of studies with different chemotherapy backbones is problematic. Potential benefits of dexamethasone include increased potency and better CNS penetration that theoretically could lead to relapse reduction compared with prednisone. However, these benefits must be counterbalanced by the potential increased risks of osteonecrosis and infectious morbidity and mortality associated with dexamethasone.32
EURO LB-02 evaluated whether replacing prednisone (60 mg/m2 per day) with dexamethasone (10 mg/m2 per day) for 28 days during induction on a NHL-BFM90 backbone would increase EFS in patients with T-LLy.28 Only patients with CNS disease received cranial radiation therapy (CRT). The overall EFS for patients with T-LLy was 82.3%. Supporting the role of dexamethasone in preventing CNS relapse, the cumulative incidence of CNS relapses at 5 years was 4% ± 1% among the 215 patients who received prednisone in the induction phase compared with 0% in the 104 patients who received dexamethasone (P = .03). However, infectious toxicity and associated treatment delays during induction were significantly higher in patients receiving dexamethasone (6.7% vs 16.5%; P = .009), leading the investigators to close the trial prematurely owing to an excessive number of toxic deaths.
COG POG 9404 was a phase 3 trial that tested whether addition of high dose methotrexate (HDMTX) to a multiagent Dana-Farber Leukemia Consortium (DFCI) backbone improved outcomes. All patients received prednisone (40 mg/m2 per day) for 21 days in induction, 1800 cGY of prophylactic CRT, and intrathecal (IT) triple therapy (ITT). Owing to high rates of neurotoxicity, investigators amended this protocol to replace ITT with IT cytarabine in induction and IT cytarabine with IT methotrexate (ITMTX) in subsequent cycles. Three of 137 (2.2%) patients with T-LLy died, though the causes of death were unclear, and 5 patients had CNS relapse (3.6%).24 Unlike patients with T-ALL who benefited from HDMTX in this trial, the difference between HDMTX and no HDMTX was not significant (EFS 81.7% ± 4.9% and 87.8% ± 4.2%; P = .38) for patients with T-LLy.
Using elements of a BFM86 backbone and 60 mg/m2 per day prednisone for 28 days in induction, the Societe Francaise d’Oncologie Pediatrique LMT96 trial sought to evaluate whether short infusions of HDMTX (10 courses of MTX dosed at 3 g/m2 over 3-hours, 7 of which are given over induction/consolidation/interphase/reinduction and 3 given during maintenance compared with 4 courses of MTX dosed at 5 g/m2 over 24-hours given during interim maintenance in typical BFM 86 backbone) could improve outcomes in patients with T-LLy. Only patients with CNS disease received CRT and no patients died of treatment-related toxicity. CNS relapses were rare (1 of 79 patients, 1.3%).25 Shortened infusions of HDMTX did not impact outcome with a 5-year EFS of 85% and overall survival of 89%. Although the COG trial A5971 included both patients with B-LLy and patients with T-LLy, the majority (86%) with disseminated disease had T-LLy.17 A5971 used a 2 × 2 factorial design to allocate patients to a modified BFM ALL regimen with intensified ITMTX or adapted BFM95 therapy with HDMTX but no ITMTX in maintenance, and also omitted CRT for patients who were CNS negative. Within each of these groups, patients were then randomized to with or without intensification with cyclophosphamide and anthracycline. All patients received 60 mg/m2 per day prednisone for 28 days in induction. There was no difference in outcomes between the arms for patients who were CNS negative (5-year EFS 80%-84%).17
AALL0434 was the first COG T-ALL trial to include patients with T-LLy. It tested whether intensification with nelarabine and HDMTX vs Capizzi-MTX (C-MTX) on an aBFM backbone would decrease on-therapy relapses.29 All patients received 60 mg/m2 per day prednisone for 28 days in induction. Based on the results of A5971, patients with T-LLy were excluded from prophylactic CRT and nonrandomly assigned to C-MTX. High-risk (HR) T-LLy, defined as ≥1% MDD in the BM at diagnosis or prior steroid therapy, were randomized to receive or not receive nelarabine during consolidation, DI, and maintenance phases. There was 1 death owing to invasive fungal infection (IFI) (1 of 299 patients, 0.33%), and 4 (1.34%) CNS relapses in patients with T-LLy. The 4-year EFS for patients with T-LLy was 84.7% ± 2.3% and the OS was 89.0% ± 2.0%.29
The most recent COG trial for T-ALL and T-LLy, AALL1231, randomized patients to receive bortezomib or not on an aBFM background.31 One goal of this study was to safely eliminate CRT for all but patients with CNS3 T-ALL/T-LLy and very HR T-ALL. To harmonize therapy among all patients, both patients with T-ALL and patients with T-LLy received a dexamethasone-based induction (6 mg/m2 per day for 28 days) in consolidation, DI, and maintenance. Although CNS relapses remained acceptably low for patients with T-LLy (1 of 101 [0.99%] patients in the bortezomib arm and 3 of 108 [2.8%] patients in the control arm), 20 (9.6%) infection-related deaths (balanced between the arms) occurred, most during induction including 11 IFIs.31 In contrast, the incidence of infection-related deaths using prednisone as the induction steroid was much lower on AALL0434 with only 1 (0.08%) fungal infection.29
Taking these data together, patients with T-LLy do not seem to benefit from use of HDMTX or prophylactic CRT. Because CNS relapse is rare in T-LLy and the benefit of dexamethasone appears to be offset by increased infectious complications, we believe the utility of dexamethasone-based treatment regimen in T-LLy is limited. Therefore, unlike patients with T-ALL for whom dexamethasone provides an advantage,11 we recommend prednisone as the steroid of choice in induction for patients with T-LLy. An additional nuance worth mentioning is that a significant number of patients with T-LLy present with large mediastinal masses that require urgent initiation of steroids before biopsy and staging workup. For these patients, we prefer prednisone/methylprednisolone (for up to 5 days) and avoidance of dexamethasone as it is more likely to obscure accurate evaluation of the CSF.
Bortezomib
Based on the recent COG trial AALL1231 with a modified aBFM backbone, the 4-year EFS for patients with T-LLy on the proteosome inhibitor bortezomib vs no bortezomib arm was 86.4% ± 4.0% vs 76.5% ± 5.1%, and 4-year OS was 89.5% ± 3.6% vs 78.3% ± 4.9%, respectively. Bortezomib did not impact EFS or OS for patients with T-ALL. Bortezomib was well tolerated for both patients with T-ALL and patients with T-LLy with similar grade 3 peripheral neuropathy between arms (52.4% vs 47.6%) and grade 4 pulmonary toxicity during induction and DI (2.6% and 3.7%) for both arms, respectively. As bortezomib is a safe and efficacious drug in newly diagnosed patients with T-LLy, we recommend incorporating it into induction and DI for all patients with T-LLy.31
Nelarabine
AALL0434 demonstrated superior 4-year disease free survival for patients with IR or HR T-ALL randomized to nelarabine vs no nelarabine.30 The primary protective effect of nelarabine appeared to be prevention of CNS relapse, which is uncommon in patients with T-LLy. Although the trial was not powered to evaluate the impact of nelarabine in patients with T-LLy, the 4-year EFS for patients with HR T-LLy treated with nelarabine (60 patients) or no nelarabine (58 patients) was similar (85.1% ± 4.8% vs 85.0% ± 4.9% [P = .834]).29 Patients with HR-T-LLy treated on the nelarabine arm vs no-nelarabine arm experienced a trend towards increased grade 1 to 4 CNS toxicity (12 vs 6 events respectively; P = .058) and a significant increase in peripheral motor (23 vs 14 events; P = .033) and sensory (26 vs 13 events, P = .005) neuropathies. In addition, financial implications of using a drug that has no proven benefit but increased toxicity for patients with T-LLy (it currently costs approximately $150,000 to treat 1 patient with 6 cycles of nelarabine) should be considered. Given this expense, as well as the rarity of CNS relapses in T-LLy and that the main benefit of nelarabine for patients with T-ALL is reduction of CNS relapses,3 we recommend reserving nelarabine for patients with T-LLy with CNS3 disease at diagnosis.
Clinical prognostic parameters
Clinical characteristics including age, bulk disease, recurrent genetic alterations, MDD, or MRD have failed to consistently identify patients with HR T-LLy and make risk stratification outside of clinical trials difficult. This remains an ongoing challenge as prognostic predictors of therapy are important to prevent overtreatment in low-risk patients and identification of patients at highest risk of fatal relapse. Here, we summarize the current status of the field.
Recurrent genetic alterations
Recent retrospective analyses identified candidate genes of prognostic relevance for T-LLy, including activating NOTCH1 and FBXW7 mutations (N/Fpos) that are involved in the regulation of many cellular processes such as early T-cell development.3,6,33-35 Mutations in NOTCH1 and/or FBXW7 at recurrent hotspots within these genes are associated with favorable treatment and outcome responses. Bonn et al evaluated 116 patients with T-LLy treated with BFM-like backbone and demonstrated the 5-year probability of EFS (pEFS) was 84% ± 5.0% in patients with NOTCH1 mutations vs 66% ± 7.0% in patients without NOTCH1 mutations. Similar results were observed in patient harboring FBXW7 mutations. Clinical features of patients with N/Fpos were similar to those without such mutations, though there was an overrepresentation of younger patients in the N/Fpos cohort.36 Other studies have similarly demonstrated a significantly higher cumulative incidence of relapse at 5 years for patients without NOTCH1 mutations.37,38 Conversely, loss of heterozygosity at chromosomal region 6q14 to 24 (LOH6q) was associated with adverse outcome and increased risk of relapse (5-year pEFS of 27% ± 9.0% in LOH6qpos vs 86% ± 3.0% for patients with LOH6qneg).36 Likewise, homozygous or biallelic mutations in the tumor suppressor gene PTEN in 17 of 113 (15%) was also a poor prognostic feature (5-year pEFS of 59% ± 12% vs 82% ± 2.0% for nonmutated cases).35 Interestingly, when combined with a NOTCH1 mutation, the favorable marker outweighs the unfavorable prognostic effect of PTENmut.35KMT2D mutations have also been associated with poor prognosis (cumulative incidence of relapse of 47% ± 17% in mutated cases compared with 14% ± 3% in nonmutated cases).39 Finally, absence of biallelic T-cell receptor gene gamma locus deletion was associated with poor outcomes in both patients with T-ALL and patients with T-LLy, though sample size in these studies remains small.40,41 Whether these markers will remain relevant as therapy evolves is unclear. Further characterization of T-LLy’s molecular landscape with the goal of incorporating prognostically significant markers into future clinical trials is critical for determining which patients will benefit from augmented initial therapy and which can avoid added toxic therapies.
MDD- and MRD-based risk stratification
COG A5971 measured MDD in the BM at diagnosis using flow cytometry and found that an MDD >1% was a negative prognostic factor (2-year EFS of 68% vs 91%; P = .031). The Italian group Associazione Italiana Ematologia Oncologia Pediatrica (AIEOP) later corroborated this conclusion, though they used a >3% cutoff and only included patients with stage I-III disease (5-year EFS of 60% vs 83%; P = .04).42,43 Based on these data, AALL0434 treated patients who were MDD positive (and steroid pretreated) with T-LLy as HR. Surprisingly, EFS and OS were the same between standard and HR patients, leading the authors to speculate that the use of C-MTX in both arms may have abrogated the prognostic significance of MDD.29,42,43 EURO-LB02 also showed no prognostic significance of MDD at 1%.44 However, changing the MDD threshold to 0.1% and combining it with genetic prognostic factors was highly predictive of relapse (5-year EFS of 37.5% vs 100% for patients with and without N/FGL and MDD ≥0.1%) in this trial.44 In the successor COG trial AALL1231, patients with BM MDD ≥1% by flow cytometry at diagnosis or steroid pretreatment were classified as intermediate risk. In the context of this therapy, MDD, as well as race, age, gender, risk group, stage and radiologic response to therapy, were not prognostic. However, EOI MRD of >0.1% in the BM by flow cytometry from patients with T-LLy who voluntarily consented to this evaluation was associated with an inferior EFS in both arms (with or without bortezomib).45 We recommend further evaluation of MDD and EOI/EOC MRD in combination with genetic alterations in future trials to clarify their prognostic significance.
Utility of PET imaging
The prognostic value of PET imaging in T-LLy remains unclear.46-49 The GRAAL-LYSA LL03 trial enrolled 76 adults with LLy and found that increased standardized uptake value (SUVmax) at diagnosis was associated with favorable prognosis but that SUVmax at EOI was not predictive of OS.46,49 A recent retrospective study by Fox et al enrolled 37 pediatric and adult patients with T-LLy treated with UKALL protocols.50 Only half of eligible patients received PET imaging at diagnosis with access to nuclear medicine or patients presenting in extremis cited as features precluding imaging. For patients able to undergo PET imaging, an SUVmax ≥10 at diagnosis was predictive of a more aggressive clinical course (OS 75% SUVmax <10 vs OS 33% SUVmax ≥10; P = .008).50 Regardless of age, all patients with a negative PET/CT scan after 1 cycle of chemotherapy survived, though the number of patients in this group was small (n = 5). Although PET positivity was not clearly defined, patients who remained PET avid after 2 cycles of chemotherapy or at the end of treatment had significantly worse OS (57% vs 0%, respectively).50 Elhussein et al prospectively enrolled 18 pediatric patients with LLy (T-LLy = 11, B-LLy = 7) treated with the St. Jude Children's Research Hospital ALL Total Therapy XV protocol.51 All patients underwent a PET/CT scan and a contrast-enhanced CT of the chest, abdomen, and pelvis at diagnosis, EOI, and at 18 weeks if EOI images were positive. Variable FDG avid lesions were seen in all patients at diagnosis and did not correlate with response. EOI CT identified 8 patients (44%) with residual masses but only 3 (16.7%) of these were PET avid. Four patients had residual masses by CT at week 18 and none were PET avid. Therapy was not intensified in any patients and there were no relapses or progressive disease.51 The specificity of postinduction PET/CT was 81% vs 50% for CT, and the negative predictive value was 87% for PET/CT compared with 80% for CT.51
Response to therapy in patients with NHL, including T-LLy, is currently based upon morphologic response in the BM and on any imaging modality including bone scan, CT chest, and/or PET/CT as determined by published international consensus guidelines.52,53 Unfortunately, imaging is frequently not safe or feasible in patients with large mediastinal masses at diagnosis. In addition, though there may be potential predictive value of PET imaging, data on its utility is limited by small numbers. AALL1231 strongly recommended but did not require a PET/CT at diagnosis, EOI, and if residual masses, at EOC for all newly diagnosed patients with T-LLy. Given the potential for false positives, we recommend histologic confirmation of residual masses at EOC before intensification of therapy.
Treatment duration
Most cooperative groups including St. Jude Children’s Research Hospital, BFM, DFCI, COG, and Nordic Society for Pediatric Hematology and Oncology (NOPHO) treat both male and female with T-LLy with equal duration.54-57 This decision is based on the observation that most relapses in patients with T-LLy occur early. For example, in patients with T-LLy enrolled onto NHL-BFM 90 and NHL-BFM 95 trials, the median time to progression or relapse was 10 months (range, 0.5-40 months) after start of treatment, which is well before the third year of maintenance therapy. Eleven of 28 (39.3%) patients suffered from disease progression or relapse during the intensive phase of frontline treatment, and only 3 of 28 (11%) patients relapsed after the end of treatment.58 Therefore, future trials should continue to treat male and female patients with T-LLy with equal duration of therapy.
Patient 2: relapsed T-LLy while on maintenance therapy
A previously healthy girl aged 6 years presented to the emergency room with abdominal pain and shortness of breath. Her complete blood count was normal, but chest x-ray and PET/CT showed a large anterior mediastinal mass with moderate pericardial effusion and scattered anterior and posterior cervical lymphadenopathy with the largest node measuring 5.6 mm × 9.7 mm with a SUVmax of 11.2 (liver SUVavg 2.2). BM aspirate demonstrated an 18% lymphoblast blast population expressing CD45, partial CD4, CD5, CD7, CD10, CD38, partial dim CD34, CD2, cytoplasmic CD3, and partial dim CD1a. CSF was negative. She started therapy on the control arm of AALL1231, which did not include bortezomib. EOI imaging and BM MRD showed a complete response (CR). Three months into maintenance therapy, she was noted to have increasing shortness of breath and a recurrent mediastinal mass. Biopsy of the mass confirmed relapsed disease. What are the treatment options for r/r disease?
Salvage options for patients with r/r T-LLy
Data to guide salvage regimens in r/r T-LLy are lacking. Bortezomib can be considered for patients who have not received it. Efficacy is demonstrated in the phase 2 COG trial AALL07P1 in which bortezomib was given with a 4-drug induction backbone. Of the 10 patients with r/r T-LLy, 3 achieved CR and 4 had a PR. Two patients who did not achieve a CR after 1 cycle subsequently attained CR after 2 cycles.59 Daratumumab, an IgG1κ human monoclonal antibody that binds to the CD38 protein, is FDA-approved for the treatment of multiple myeloma and can also be considered.60 CD38 is highly expressed on the surface of T-cell lymphoblasts, and its expression persists after treatment with chemotherapy.61 Daratumumab was recently investigated in children and young adults with r/r T-ALL or T-LLy (NCT03384654), where it was administered with standard BFM induction and consolidation chemotherapy. There were 4 CRs and PR out of 10 response evaluable patients with T-LLy.62 In addition, the NCI trial NCT04972942 will test immunotherapy with daratumumab after allogeneic hematopoietic stem cell transplantation (HSCT) in patients with r/r T-ALL/LLy. Nelarabine is another option and has been given in combination with etoposide and cyclophosphamide, though response rates are comparable with those seen with single agent nelarabine.63 Alternatively, a recent case study reported 2 patients with relapsed T-LLy who achieved CRs with a combination of daratumumab, nelarabine, dexamethasone, and asparaginase.64 Although data in T-LLy are limited, venetoclax and CDK4/6 inhibitors (ribociclib, palbociclib) have demonstrated activity in T-lineage disease and could also be considered.65,66 The safety and preliminary efficacy of venetoclax with low-dose navitoclax in combination with chemotherapy was assessed in a phase 1 dose-escalation study (NCT03181126) that included both adult and pediatric patients with r/r ALL and LLy.67 In this heavily pretreated population, data were encouraging as they demonstrated an overall CR rate of 59.6% (28/47) for all patients and 75% (9/16) among pediatric patients. Finally, Lu et al recently published the results of a phase 1 trial using CD7-targeted chimeric antigen receptor (CAR) T cell for patients with T-ALL and T-LLy (NCT04572308). Their novel approach was able to overcome T-cell fratricide by minimizing accessible CD7 epitopes using naturally selected 7CAR (NS7CAR). On day 28, all 6 of 6 patients (100%) achieved CR with incomplete hematologic recovery (CRi) and MRD-negativity. Five patients received consolidative allogeneic HSCT at a median time of 57.5 (42-92) days after infusion, with 1 death secondary to grade 3 acute graft-versus-host disease (patient received donor-derived CAR T cells).68
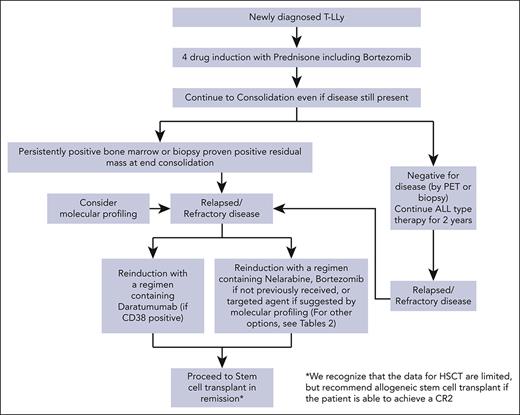
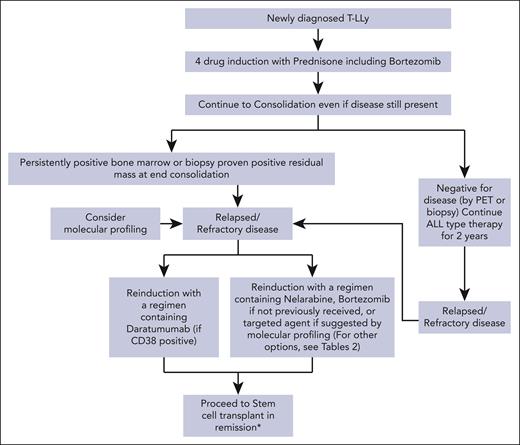
For patients with r/r T-LLy, if a clinical trial is not available, we recommend treating with bortezomib if not previously given or daratumumab as first-line therapy. Nelarabine, other experimental agents, or if available, a CD7 CAR T trial are alternative options. Molecular profiling at relapse and incorporation of an appropriate targeted agent, if available, is another consideration. Patients that achieve a CR should immediately receive an allogeneic transplant with best available donor. A treatment algorithm for frontline and salvage therapy for patients not enrolled in a clinical trial is presented in Figure 2.
Treatment algorithms for children, adolescents, and young adults with newly diagnosed and r/r T-LLy. ∗The data for HSCT are limited, but allogeneic stem cell transplant is recommended if the patient is able to achieve a CR2.
Treatment algorithms for children, adolescents, and young adults with newly diagnosed and r/r T-LLy. ∗The data for HSCT are limited, but allogeneic stem cell transplant is recommended if the patient is able to achieve a CR2.
Criteria to transplant
The prognosis for r/r T-LLy is dismal12 (Table 2). These poor outcomes primarily reflect the challenge in getting patients into CR before HSCT. As discussed above, validated prognostic factors to identify patients that would benefit from early HSCT and data to guide management of patients with residual mass, PET positivity, and/or persistent MRD at EOC are limited and often conflicting. Nonetheless, given the poor salvage rates for patients with r/r T-LLy, we recommend attempting to achieve a CR with alternative therapies followed by consolidative allogeneic HSCT from best available donor for patients with persistent BM disease or histologically confirmed residual masses at EOC as well as for all patients that relapse. Superiority of allogeneic transplant is supported by lower progression rate and improved EFS compared with that of autologous transplant.70,74,75
Conclusion and future directions
Ongoing studies are underway to refine our understanding of T-LLy in hopes of providing reliable clinical prognostic markers to best treat those with newly diagnosed and at risk for r/r disease. In the era of targeted molecular therapy and immunotherapy, retrospective and prospective next-generation-sequencing of T-LLy samples will be important to define disease drivers that are unique to T-LLy. Finally, preliminary research on prior COG trials demonstrates outcome differences in patients with T-LLy of various race/ethnicities (unpublished data), therefore emphasis on childhood disparities research in relation to biologic and social constructs need further evaluation. Although much work remains to be done, steady improvements in outcome for patients with T-LLy have been made through international collaborative research and understanding of this disease continues to advance.
Acknowledgments
The authors thank members of the Children’s Oncology Group Non-Hodgkin’s Lymphoma Disease Committee for helpful discussions. The authors thank Carl Allen, Kaitlin Devine, Nader Kim El-Mallawany, Paul Harker-Murray, Mandy Termuhlen, and Birte Wistinghausen for critical reading of the manuscript. The authors thank Catherine Bollard, Mitchel Cairo, Thomas Gross, Robert Hayashi, and Megan Lim for their mentorship and support.
Authorship
Contribution: S.J.S.L., J.B.F., and M.L.H. wrote the manuscript.
Conflict-of-interest disclosure: M.L.H. has served on external advisory boards for Novartis and Sobi Pharmacueticals. The remaining authors declare no competing financial interests.
Correspondence: Stephanie J. Si Lim, University of Hawai'i, 701 Ilalo St, Honolulu, HI 96813; e-mail: sjys@hawaii.edu.
References
Author notes
All data are available on request from the corresponding author, Michelle L. Hermiston (michelle.hermiston@ucsf.edu).