Cytokines are key proinflammatory messenger molecules that help to regulate the immune system and inflammatory responses. They are well established in the pathogenesis of myeloproliferative neoplasms (MPNs), including in advanced stages of disease, such as myelofibrosis (MF).1 In this issue of Blood, Dunbar et al identify the chemokine (C-X-C motif) ligand 8 (CXCL8)/CXCR2 cytokine signaling axis as a key mediator of bone marrow fibrosis in MPNs, providing a therapeutically tractable avenue to prevent or treat this lethal complication of MPNs.2
The classic MPNs include essential thrombocythemia (ET), polycythemia vera (PV), and primary MF, characterized by the proliferation of mature, functional myeloid lineage cells, with a propensity to transform into more advanced disease, including secondary MF or acute leukemia. MF is associated with increased constitutional symptom burden, splenomegaly, and bone marrow failure, and it is therefore associated with adverse prognosis and reduced survival. These diseases are causally linked by recurrent activating mutations in the erythropoietin and thrombopoietin signaling pathway, leading to constitutionally activated JAK-STAT signaling, which, in turn, drives a proinflammatory state.3 Additional mutations in epigenetic pathways may further drive proinflammatory signaling and the development of MF.4-6 Patients with advanced MF exhibit increased levels of cytokines that correlate with symptom burden. Moreover, these cytokine levels are diminished by inhibitors of JAK-STAT signaling, such as ruxolitinib.7 However, this complex milieu of cytokines also regulates normal patient immune function and, therefore, approaches leading to global suppression of cytokine signaling may result in significant morbidity.8
To determine the key cytokines that were responsible for the myelofibrotic phenotype, the authors used primary human samples from patients with MPN and performed single-cell cytokine profiling. CXCL8 (also known as interleukin 8) has been previously identified as a key circulating chemoattractant cytokine that is increased in patients with MF.9 Consistent with these previous descriptions, the authors were able to demonstrate a gradient of CXCL8 expression in patients with MPN, with limited expression in early-phase disease, such as ET and PV, and greater expression in some patients with MF. The CD34+ hematopoietic stem and progenitor cell populations (HSPCs) from these MF samples showed enhanced sensitivity to stimulation with exogenous CXCL8. Next, bulk CD34+ cells were examined using RNA sequencing to determine gene expression, again showing activation of inflammatory pathways, including tumor necrosis factor-α signaling, NF-κB signaling, and toll-like receptor (TLR) signaling. These CD34+ HSPCs showed increased areas of chromatin accessibility within these pathways, demonstrating that these cells are primed for proinflammatory signaling.
To provide robust functional validation of their findings, the authors examined therapeutic approaches to modulate the CXCL8/CXCR2 axis. Although mice lack CXCL8, mouse CXCR2 shares substantial homology with humans, and human CXCL8 can signal through CXCR2. Using the retroviral overexpression model of the human oncogene, hMPLW515L, that causes a rapid, fatal MF,10 the authors showed that by deleting CXCR2 in HSPCs, the recipient mice have an attenuated MF disease phenotype characterized by reduced bone marrow fibrosis, improved overall survival, and reduced inflammatory signaling via TLRs. Loss of CXCR2 did not appear to substantially inhibit bone marrow homing or engraftment of normal HSPCs. They subsequently used reparixin, a CXCR1/2 inhibitor, in the hMPLW515L MF model. This compound was able to reduce white cell and platelet count, bone marrow megakaryocyte formation, and, more important, bone marrow reticulin fibrosis. When used in combination with ruxolitinib, there appeared to be further benefit in terms of preventing bone marrow fibrosis. These data validate this approach to treat MF in these models. Several future questions remain, including what is the toxicity and impact on normal patient immune function of this compound, or other approaches, such as monoclonal antibodies targeting CXCL8/CXCR2 signaling? If this approach is to be used clinically, is it best to be used as monotherapy or in combination with JAK inhibitors? Finally, are the responses to treatment comparable between the distinct molecular subgroups of MF, driven by constitutively active myeloproliferative leukemia, JAK2, or mutant calreticulin?
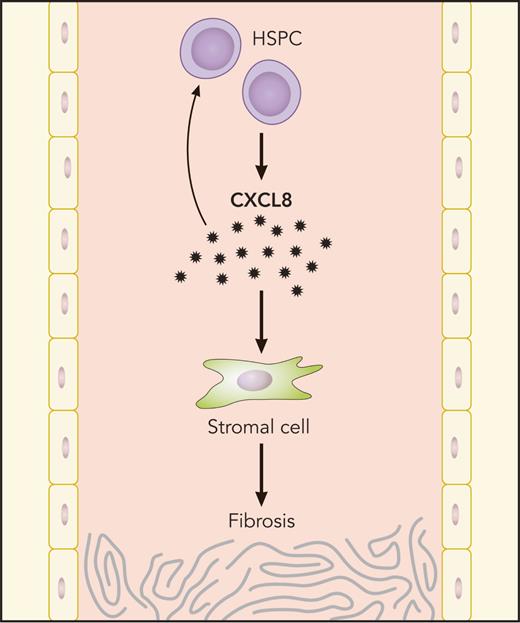
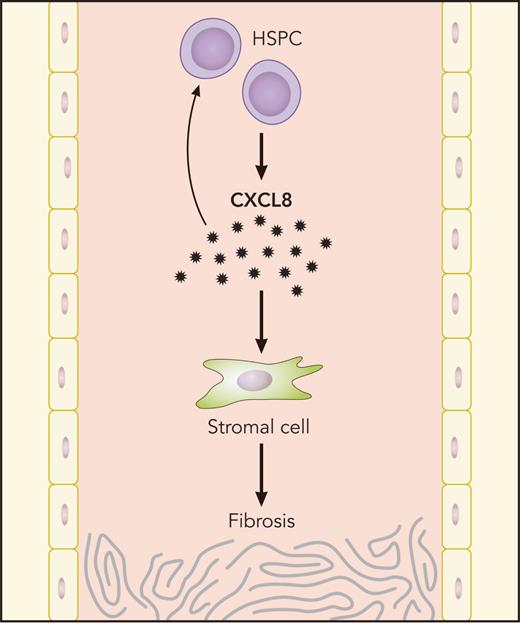
In summary, this work used complementary approaches from primary human samples and mouse models of MPN to identify a specific cytokine signaling axis, CXCL8/CXCR2, that regulates inflammatory signaling in patients with MF (see figure). Targeting this axis appears to be effective in several models of MF, and this work suggests that future clinical trials should be considered to target CXCL8/CXCR2 signaling in patients with MF. These results provide promising data in MF, a disease in which clinicians and patients have limited therapeutic options and survival remains poor.
MF HSPCs generate CXCL8, which, in turn, propagates bone marrow fibrosis through autocrine HSPC stimulation and effects on stromal cells. Professional illustration by Patrick Lane, ScEYEnce Studios.
MF HSPCs generate CXCL8, which, in turn, propagates bone marrow fibrosis through autocrine HSPC stimulation and effects on stromal cells. Professional illustration by Patrick Lane, ScEYEnce Studios.
Conflict-of-interest disclosure: The author declares no competing financial interests.