Clarke et al1 report in this issue of Blood that loss of 1 allele of Myb in mice results, upon aging, in increased myeloproliferation and subsequent development of a range of myeloid neoplasms (MNs). It is well established that the MYB transcription factor, encoded by the MYB (aka c-Myb) gene, is essential for normal hematopoiesis and that activation of MYB leads to myeloid and other leukemias in several animal species and occasionally in humans.2 Moreover, initiation and progression of acute myeloid leukemia (AML) is critically dependent on MYB as shown by numerous in vitro and in vivo loss-of-function studies (eg, as reviewed elsewhere3,4). It is surprising, therefore, that a previous study from the Frampton group5 and now their new report have suggested that partial loss of MYB activity can also predispose to MNs, including AML.
The authors found that cell numbers were elevated in all myeloid compartments from the hematopoietic stem cell (HSC)-enriched Kit+Sca+Lineage− (KSL) population through to myeloid progenitors and mature monocytes and granulocytes in Myb+/− mice. By deleting a single Myb allele in mice of different ages (2 vs 12 months) and following the time at which these MNs appear, the authors showed that appearance of MNs is age dependent but is initiated by Myb deficiency. Transplantation studies revealed that these phenotypes originate at least partially in compromised HSCs from Myb+/− mice.
Clarke et al then explore the nature of the hematopoietic defects in Myb+/− mice, and possible mechanisms leading to age-dependent development of MNs, by examining differential gene expression in the KSL population across young and old, wild-type (WT) and Myb+/− animals. Functional classification of genes differentially expressed between WT and Myb+/− mice showed enrichment of genes involved in protein synthesis and degradation, RNA splicing, and cell cycle or DNA replication. The authors focus on genes encoding ribosomal and particularly proteasome components and found, by comparison with published studies of direct MYB target genes in K562 myeloid leukemia cells, that a number of the latter genes appeared to be negatively regulated by MYB. Direct measurement confirmed that proteasome activity was indeed elevated in Myb+/− HSCs, as was total protein synthesis (in older animals); in totality these changes imply altered protein turnover and possible proteostatic stress.
These observations raise several interesting questions, but I will focus here on 2 of these: first, how does Myb deficiency and the consequences thereof, as identified by Clarke et al, lead to enhance myeloproliferation and MN? And second, what is the potential relevance for human myeloid disease?
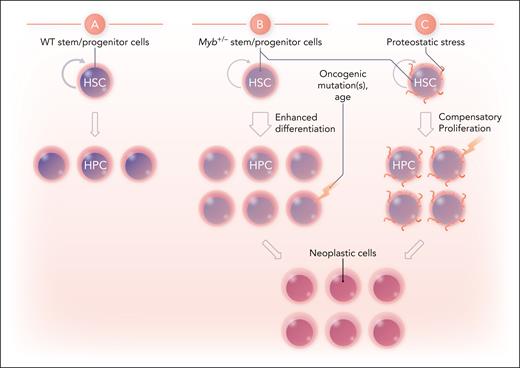
One key activity of MYB is to restrict hematopoietic differentiation, as shown for example by the nature of its oncogenic activity, by the effects of MYB knockdown on leukemia cells, and conversely by its downregulation during late stages of hematopoietic cell maturation. Thus it is conceivable that partial Myb deficiency per se could promote differentiation of HSCs and lead to increased numbers of myeloid progenitors, as reported by the authors (this might also contribute to the functional deficiency of these HSCs through diminished long-term self-renewal). As well as accounting for the increased cell numbers across the myeloid compartment, the increase in progenitor numbers could provide an increased target population for subsequent oncogenic events leading to the observed MNs (see figure panels A and B).
Possible mechanisms for promotion of myeloid neoplasias by partial Myb deficiency. (A) WT HSCs balance self-renewal and differentiation to hematopoietic progenitor cells (HPCs). (B) Myb deficiency may enhance differentiation to myeloid HPCs, providing an increased target population for subsequent oncogenic events. (C) Proteostatic stress resulting from altered expression of MYB-regulated genes leads to compensatory proliferation or other prooncogenic changes favoring subsequent oncogenic events. Professional illustration by Somersault18:24.
Possible mechanisms for promotion of myeloid neoplasias by partial Myb deficiency. (A) WT HSCs balance self-renewal and differentiation to hematopoietic progenitor cells (HPCs). (B) Myb deficiency may enhance differentiation to myeloid HPCs, providing an increased target population for subsequent oncogenic events. (C) Proteostatic stress resulting from altered expression of MYB-regulated genes leads to compensatory proliferation or other prooncogenic changes favoring subsequent oncogenic events. Professional illustration by Somersault18:24.
An alternative, but importantly not mutually exclusive, possibility proposed by the authors lies in the increased proteasomal activity resulting from derepression of proteasome subunit genes and broadly altered proteostasis in Myb+/− HSCs. The observed increase in protein synthesis (consistent with activation of genes in ribosomal pathways) could be a compensatory cellular response. In any case, these dual effects on proteostasis may lead to a stress response, which in turn may directly or indirectly enhance the likelihood of subsequent oncogenic events. There are multiple precedents for this; genetic lesions that compromise hematopoiesis by promoting replicative stress or DNA damage (eg, Fanconi anemia) or nucleolar stress (eg, Diamond-Blackfan anemia) both increase the risk of developing AML.6,7 Although it is probably fair to say that the precise mechanisms by which these conditions promote leukemia are not clear, they may involve compensatory proliferative responses or selection for loss of stress response pathways (eg, p53 activation in the case of Fanconi anemia8). Again, this in turn could provide an enlarged or more susceptible target population for subsequent leukemogenic events (figure panel C); in this context, it would be interesting to examine direct markers of stress and the response thereto in the Myb+/− mice.
It would also be of interest to examine the acquired oncogenic driver mutations that are present in MNs that arise in Myb+/− mice. It is highly unlikely that these have lost dependency on Myb, and indeed Clarke et al found that MYB expression in these was at least equivalent to that in WT cells. So, might there be selection in these MNs for drivers that directly or indirectly increase Myb expression?
Finally, we should consider the implications for human myeloid disease. A single-nucleotide polymorphism (SNP) in an intergenic region near MYB is associated with risk of developing myeloproliferative neoplasias (MPNs).9 Moreover, this SNP appears to result in a substantial reduction in MYB expression in myeloid and erythroid progenitors.9 As Clarke et al point out, this result is consistent with the findings from their mouse model, and indeed they confirm that MYB levels are modestly reduced in human HSCs carrying the variant (ie, MPN-associated) allele. Taken together, these data strengthen the case that a moderate reduction in MYB expression can predispose or contribute to development of MNs and that, given the proleukemic roles of MYB mentioned previously, precise regulation of MYB expression is critical to maintain normal hematopoiesis while preventing neoplasia.
Conflict-of-interest disclosure: T.J.G. declares no competing financial interests.