Abstract
By overcoming chemotherapeutic resistance, chimeric antigen receptor (CAR) T cells facilitate deep, complete remissions and offer the potential for long-term cure in a substantial fraction of patients with chemotherapy refractory disease. However, that success is tempered with 10% to 30% of patients not achieving remission and over half of patients treated eventually experiencing relapse. With over a decade of experience using CAR T cells in children, adolescents, and young adults (AYA) to treat relapsed/refractory B-cell acute lymphoblastic leukemia (B-ALL) and 5 years since the first US Food and Drug Administration approval, data defining the nuances of patient-specific risk factors are emerging. With the commercial availability of 2 unique CD19 CAR T-cell constructs for B-ALL, in this article, we review the current literature, outline our approach to patients, and discuss how individual factors inform strategies to optimize outcomes in children and AYA receiving CD19 CAR T cells. We include data from both prospective and recent large retrospective studies that offer insight into understanding when the risks of CAR T-cell therapy failure are high and offer perspectives suggesting when consolidative hematopoietic cell transplantation or experimental CAR T-cell and/or alternative immunotherapy should be considered. We also propose areas where prospective trials addressing the optimal use of CAR T-cell therapy are needed.
Introduction and background
The first child to receive CD19-targeted chimeric antigen receptor (CAR) T cells for B-cell acute lymphoblastic leukemia (B-ALL) recently celebrated an important milestone: a decade in remission, vividly demonstrating that long-term, durable remissions can occur in highly chemotherapy refractory patients.1,2 There are currently 2 CD19 CAR T-cell constructs approved by the US Food and Drug Administration (FDA) for relapsed/refractory (r/r) B-ALL. Tisagenlecleucel (Kymriah), which incorporates the 4-1BB costimulatory domain, received FDA approval in 2017 for children and young adults ≤25 years,3,4 and brexucabtagene autoleucel (Tecartus), which incorporates the CD28 costimulatory domain, received FDA approval in 2021 for those ≥18 years with r/r B-ALL.5,6
Emerging data encompassing both commercial and academic CAR T-cell constructs has led to the identification of risk factors for both response and long-term remission. These data can inform how we approach individual patients to optimize outcomes. Here, we describe in detail pre- and postinfusion risk factors associated with (1) nonresponse, (2) post-CAR relapse and event free-survival (EFS), and (3) long-term durable remission. We then propose a framework incorporating these risk factors to optimize CD19 CAR T-cell outcomes in children and adolescents and young adults, recognizing that rigorous trials are needed to validate our approaches. Through an evolving patient case study, we illustrate key points in the therapeutic path associated with CD19 CAR T-cell therapy.
Case: a 19-year-old male presents with first relapse of B-ALL 6 months after completion of therapy. At diagnosis, he was high-risk by age with normal cytogenetics and no molecularly targetable lesion, which did not change with relapse. He is treated with 4-drug reinduction, followed by blinatumomab, but has persistent CD19+ disease. Plans are made to proceed with CD19 CAR T cells. HLA typing is initiated to identify potential donors, but the family expresses a desire to avoid an allogeneic hematopoietic stem cell transplantation (HSCT).
Preinfusion considerations
Construct selection
Given this patient’s age and nonresponse to 2 lines of post-relapse therapy, he meets the indications to receive either of the 2 commercially available CAR T-cell constructs. Without head-to-head comparisons of tisagenlecleucel to brexucabtagene autoleucel and limited “real-world” experience with the latter, data comparing long-term outcomes for those from 18 to 25 years who are eligible for both are not available. The ZUMA-3 registration study for brexucabtagene autoleucel had a median age of 40 years (interquartile range, 28-52 years), limiting our ability to assess use in younger adults.6 The pediatric study (≤21 years) of brexucabtagene autoleucel is ongoing (NCT02625480, ZUMA-4).7
With the above limitations, a key factor in construct selection is based on CAR T-cell persistence and treatment goals. Although tisagenlecleucel has established persistence and demonstrated the potential for durable remission without HSCT,8 the shorter persistence of CD28-containing CD19 CAR T-cell constructs (brexucabtagene autoleucel and its predecessor axicabtagene autoleucel9) and poor long-term survival without consolidative HSCT in this age group are critical considerations. We recommend consolidative HSCT for those in remission after receiving a CD28 CAR T-cell construct, particularly in HSCT-naïve patients.10 Thus, for families wishing to avoid or who are ineligible for HSCT, tisagenlecleucel would be preferable. Longitudinal data, preferably a comparative trial, would be needed to compare outcomes of tisagenlecleucel with brexucabtagene autoleucel if CAR T-cell therapy was used as a planned bridge to HSCT.
In addition, although refractory disease after relapse is an indication for either construct, the FDA label for tisagenlecleucel11 is restricted to second or later relapse, because the global registration ELIANA trial (NCT02435849) only included first relapse patients who were refractory to reinduction.4 Although the label for brexucabtagene autoleucel states that it is for adults with relapsed or refractory B-ALL,12 the international, multicenter phase 1 to 2 ZUMA-3 registration trial (NCT02614066) enrolled patients in first relapse only if the initial remission was <12 months.6,13 It should be noted that current therapies are insufficient for younger patients with a high- or intermediate-risk first relapse (eg, <36 months after diagnosis or late first relapse with minimal residual disease [MRD] positivity after reinduction). On the most recent phase 3 Children’s Oncology Group trial for first relapse of B-ALL (AALL1331), the 24-month intent-to-treat EFS was only 25% for those with a high-risk first relapse.14 The optimal use of CD19 CAR T cells in pediatric patients with a high- or intermediate-risk first relapse needs to be further explored, as it may lead to better outcomes than current approaches.
Previous blinatumomab use
Our patient received the CD3-CD19 bispecific T-cell engager blinatumomab, which is FDA-approved for the treatment of r/r B-ALL.15,16 Because of the concern that CD19 modulation with blinatumomab could impair subsequent CD19 CAR T-cell outcomes,17-19 the global registration trial of tisagenlecleucel excluded blinatumomab-exposed patients.4 Therefore, until recently, there was minimal data to shed light on the impact of sequential CD19-directed immunotherapy.
To address this gap, the association of prior blinatumomab exposure with CD19 CAR T-cell outcomes was assessed among 420 patients from 7 pediatric centers through the CAR-multicenter analysis (CAR-MA).20 The analysis revealed that blinatumomab exposure alone was not a risk factor for worse outcomes. However, nonresponse to prior blinatumomab was associated with lower complete remission (CR) rates (65% vs 93%) and shorter EFS, relapse-free survival (RFS), and overall survival (OS) (Table 1), with relapse rates exceeding 50% by 6 months (Figure 1A). These results suggest that blinatumomab nonresponse may serve as a proxy for identifying patients with either intrinsic resistance to CD19-targeting, T-cell dysfunction, or other high-risk features.20 However, use of blinatumomab and/or nonresponse should not be a contraindication to the use of CAR T cells, particularly as most patients refractory to blinatumomab do achieve a CR with CAR T-cell therapy.
Impact of previous blinatumomab treatment and disease burden on EFS following CD19 CAR T cells and OS following relapse. EFS, defined as the time from CD19 CAR T-cell infusion to one of the following events: no response, relapse, or death. (A) EFS stratified by blinatumomab-naïve patients (no blina—teal) vs blinatumomab-exposed patients who achieved a CR to blinatumomab (blina-CR—blue) vs blinatumomab-exposed patients who did not achieve a CR to blinatumomab (blina-no CR—red). P values for EFS curves: .59 (no blina vs blina-CR); .01 (blina-CR vs blina-no CR); .001 (no blina vs blina-no CR). (B) EFS stratified by high disease burden (≥5% bone marrow blasts—blue [high]) vs low disease burden (<5% bone marrow blasts—red (low). (C) OS following relapse, stratified by relapse immunophenotype. Median OS for CD19+ relapse was 18.9 months (95% CI, 11.2-27.0 months). Median OS for CD19− relapse was 9.7 months (95% CI, 6.9-15.9 months). Median OS for LS was 3.7 months (95% CI, 1.2-7.0 months). Red: CD19+; blue: CD19−; green: LS. CI, confidence interval; LS, lineage switch. (A-B) Reproduced with permission from Wolters Kluwer Health, license number 5363041439390, from Myers et al20; (C) reproduced with permission from Elsevier, license number 5363050768789, from Lamble et al.23
Impact of previous blinatumomab treatment and disease burden on EFS following CD19 CAR T cells and OS following relapse. EFS, defined as the time from CD19 CAR T-cell infusion to one of the following events: no response, relapse, or death. (A) EFS stratified by blinatumomab-naïve patients (no blina—teal) vs blinatumomab-exposed patients who achieved a CR to blinatumomab (blina-CR—blue) vs blinatumomab-exposed patients who did not achieve a CR to blinatumomab (blina-no CR—red). P values for EFS curves: .59 (no blina vs blina-CR); .01 (blina-CR vs blina-no CR); .001 (no blina vs blina-no CR). (B) EFS stratified by high disease burden (≥5% bone marrow blasts—blue [high]) vs low disease burden (<5% bone marrow blasts—red (low). (C) OS following relapse, stratified by relapse immunophenotype. Median OS for CD19+ relapse was 18.9 months (95% CI, 11.2-27.0 months). Median OS for CD19− relapse was 9.7 months (95% CI, 6.9-15.9 months). Median OS for LS was 3.7 months (95% CI, 1.2-7.0 months). Red: CD19+; blue: CD19−; green: LS. CI, confidence interval; LS, lineage switch. (A-B) Reproduced with permission from Wolters Kluwer Health, license number 5363041439390, from Myers et al20; (C) reproduced with permission from Elsevier, license number 5363050768789, from Lamble et al.23
CD19 expression considerations
Although CD19 escape was not found to be the primary mechanism of inferior outcomes in blinatumomab-exposed patients who retained robust CD19 expression,20 for those whose CD19 expression became dim following blinatumomab, the risk of antigen-negative relapse after CD19 CAR T cells in the CAR-MA study was particularly high.20 Of note, the incidence of pediatric patients becoming fully CD19− following blinatumomab is unknown. Given the increasing use of blinatumomab, additional studies assessing CD19 expression over time and the effect of sequential CD19 targeting with different agents are warranted.
In addition, for blinatumomab-naïve patients, the risk of relapse with CD19− disease remains of concern, indeed in the ELIANA registration study, most relapses were CD19−.4 Recent studies show an association between high disease burden and emergence of CD19− relapse.22,23 Whether this reflects an increased risk of a CD19− clone being present in a high disease burden scenario or whether high disease burden results in the persistence of functional CAR T cells and, over time, more genetic pressure for CD19 escape warrants future study.
Additional risk factors
Baseline patient characteristics, including age, sex, and history of trisomy 21, do not appear to affect outcomes.20,24,27,37 Surprisingly, leukemia cytogenetics also generally do not affect outcomes (Table 1). In a large, retrospective analysis from Children’s Hospital of Philadelphia (n = 231) patients with high-, intermediate-, and low-risk cytogenetics all had similar RFS, illustrating the remarkable ability of CAR T cells to overcome biological risk factors that portend a poor response to chemotherapy.38 A smaller study from 2 centers in France (n = 51) demonstrated similar results.22
Several specific cytogenetic lesions, however, deserve additional consideration. Patients with KMT2A rearrangement (KMT2Ar) have similar RFS compared with other cytogenetic groups after CAR T-cell therapy; however, an increased risk of myeloid lineage switch has been noted (reported populations have been mostly infant ALL).23,38,39 Unfortunately, survival after a lineage switch is dismal, leading to an inferior OS. In addition, poor long-term outcomes have been reported in samples of patients with TP53 mutations24 or hypodiploid B-ALL,20,38 but individual cohorts have been too small to draw firm conclusions.
Finally, an increased quantity of prior therapy, reflective of more refractory disease and potentially dysfunctional T cells, has been associated with worse CD19 CAR outcomes. A Pediatric Real World CAR Consortium (PRWCC) study found that a greater number of lines of prior therapy (measured as a continuous variable) was independently associated with worse OS with a hazard ratio (HR) of 1.4 (P = .022).27
Case (continued): given the desire to avoid allogeneic HSCT, the patient moved forward with tisagenlecleucel. With confirmed CD19+ expression following blinatumomab and an adequate lymphocyte count, plans were made to proceed with apheresis. However, with 26% of bone marrow blasts present, bridging therapy was deemed necessary.
Getting to CAR T cells
Access
Despite the commercial availability of CAR T cells, there are many barriers to access, and making an early referral, obtaining insurance approval, and potentially transitioning a patient to another center can be time-intensive, leading to delays in therapy. Insurance approval must be obtained both for apheresis and CAR T-cell therapy. This can be especially challenging in situations where patients are at very high risk and centers attempt to preemptively collect before relapse, or when patients need to move across state or international borders for therapy. Early referral for CAR T-cell therapy may help address these issues and may also be beneficial for subgroups with chemotherapy refractory disease, allowing earlier collection of healthy T cells that could lead to better outcomes.40
Leukapheresis
The first step of CD19 CAR T-cell manufacturing is the collection of autologous peripheral blood mononuclear cells by leukapheresis. Optimal collection practices may improve the outcome as T-cell quality affects CAR T-cell functionality. Compelling data demonstrate that T-cell populations enriched for early lineage cells have improved expansion and that early lineage cells are selectively depleted with continued courses of chemotherapy.40,41 Hence, if CD19 CAR T cells are being considered, we recommend collection as early as possible, before initiation of chemotherapy for relapse when feasible. In addition, preemptive apheresis may be considered for very high-risk patient subsets (eg, infant ALL MRD+ after induction).
The timing of apheresis involves consideration of multiple factors, including disease progression and time from previous therapy (Table 2). Manufacturing standards for tisagenlecleucel require at least 1 × 109 CD3+ cells, with rare exceptions for infants and very small children. Therefore, a minimum absolute lymphocyte count of ≥300 cells per μL or a CD3+ cell count of 100 to 200 cells per μL is recommended, with higher values preferable. Although there may be a narrow window for collection, collection is generally not advisable in patients with rapid disease progression and elevated circulating blasts (white blood cell > 20-40 000/μL), as high blast counts can interfere with manufacturing,42 although the threshold below which apheresis is acceptable has yet to be defined and may change with improvements in manufacturing and selection methodologies.43 Importantly, such patients can often be successfully collected after resolution of hyperleukocytosis with additional chemotherapy.
Bridging therapy
Once a patient undergoes apheresis, bridging therapy during the manufacturing period needs to be considered based on the extent of the underlying disease and the anticipated time to CAR T-cell infusion. The intensity of this therapy should be individualized to avoid toxicities that could delay or preclude CAR T-cell infusion. Recognizing the association of high disease burden with poor CAR T-cell outcomes,20,27,30 the desire to give more intensive therapies to reduce the preinfusion disease burden has to be balanced against the risk of infection, organ damage, or cytopenias, and the likelihood that intensification will not result in CR in highly chemorefractory disease. In addition, 1 study showed the importance of having at least 15% of bone marrow cells (normal B cells or blasts) expressing CD19+ before therapy to optimize CAR T-cell expansion and durability.32 Therefore, for patients in remission or with central nervous system (CNS)–only disease, minimal or no bridging therapy to allow for normal B-cell expansion in the peripheral blood is a reasonable approach. Various bridging therapies, including chemotherapy, radiation therapy, and immunotherapy, have been used before CAR T cells, and specific considerations are summarized in Table 2.44,45,47
Case (continued): after successful apheresis, the patient receives maintenance-style chemotherapy during CAR T-cell manufacturing. Preinfusion marrow disease burden was 30% without evidence of CNS or testicular involvement.
Pre–CAR T-cell therapy/time of infusion risk factors
Marrow disease burden
High bone marrow disease burden at the time of infusion is the most important risk factor associated with toxicity.49-51 In addition, high disease burden is also highly prognostic for worse long-term RFS.20,27-30 Unfortunately, there is not a standard definition for “high disease burden.” The initial analyses of cytokine release syndrome (CRS) after tisagenlecleucel identified 40% of bone marrow blasts as the threshold for severe CRS risk.50,52 Most analyses of CAR response identified 5% of bone marrow blasts as the threshold for increased relapse and worse RFS.20,27,29,30 The association of high disease burden with inferior outcomes is striking. In a report from the PRWCC (n = 185), patients with bone marrow blasts >5% had 12-month EFS and OS rates of 31% and 58%, compared with 70% and 85% for those with low disease burden, respectively (P < .0001).27 A report from the CAR-MA study (n = 420) further demonstrated that these poor outcomes (Figure 1B) were specifically driven by a higher cumulative incidence of CD19− relapse (HR 5.2, P < .001).23 These compelling findings may identify a subset for whom CAR T cells may not lead to long-term, durable remissions and where post–CAR T-cell consolidative HSCT could be considered. Although disease burden is a potentially modifiable variable, intensifying bridging therapy may be counterproductive and lead to more toxicity; a careful study of this issue would be worthwhile.
CNS disease burden
For CNS disease, because of concerns about immune effector cell–associated neurotoxicity syndrome (ICANS), most early CD19 CAR T-cell trials excluded patients with bulk CNS tumors or CNS3 disease. More recently, however, data have been published showing that patients with CNS disease who undergo CAR T-cell therapy have similar outcomes to those without CNS disease, with no increase in severe ICANS,31,53 but mixed results on the risk of relapse.54 Based on these data and our clinical experience, for patients with CNS disease at referral, we recommend: (1) treating patients with intrathecal and sometimes CNS-targeted systemic therapy to ensure stable/responding disease at the time of CAR T-cell infusion; (2) proceeding cautiously (following optimal preinfusion treatment for risk mitigation) in patients with persistent CNS parenchymal lesions, as this may increase ICANS risk based on emerging data from CNS tumors and CAR T cells55 and until more data in B-ALL is available; (3) allowing a 4- to 8-week interval from cranial radiation to infusion for patients requiring focal or more general cranial irradiation to provide distance from potential side effect of CNS radiation (eg, somnolence syndrome)56 that could mimic symptoms of ICANS; and (4) ensuring no acute or worsening neurologic toxicity at the time of infusion. The use of antiepileptics in patients with preexisting CNS disease or prior neurotoxicity (eg, methotrexate toxicity) should be considered to mitigate risk of seizures.57
Non-CNS extramedullary disease (EMD)
For non-CNS EMD, the available data is limited. A large retrospective analysis found that active EMD during infusion was independently associated with worse EFS (HR 1.9, P = .01).20 A PRWCC analysis found that in 15 patients with non-CNS EMD, 10 achieved a CR, and 6 of the 10 subsequently relapsed. Consequently, only 4 of 15 patients remained event-free during the follow-up period.31 A retrospective analysis from the NCI identified limitations in the eradication of non-CNS EMD with CAR T cells, despite concurrent marrow responses, highlighting the important role of positron emission tomography scans for monitoring.58 Reducing bulky EMD with radiation or chemotherapy before infusion may improve overall outcomes, but residual disease should not preclude CAR T-cell treatment. Given little data regarding CAR T-cell trafficking to and efficacy with testicular disease, we recommend considering definitive treatment of testicular disease before infusion.
Lymphodepletion
Lymphodepletion is essential to provide an in vivo environment that will enhance the expansion, function, and persistence of CAR T cells, and most regimens include fludarabine and cyclophosphamide (Table 2), based on data from the pivotal CD19 CAR trials.47 Recent data suggests there may be room to further optimize these regimens. Two retrospective studies found that suboptimal fludarabine exposure, defined as a measured or estimated area under the curve <13.8 to 14.0 mg × h/L, was associated with increased relapse risk and a higher probability of premature loss of B-cell aplasia (BCA).33,34 Although there is insufficient data to change standard lymphodepletion regimens, optimizing lymphodepleting agents is an area where prospective, comparative trials may lead to improved outcomes, especially in light of recent drug shortages.59
Case (continued): the patient received tisagenlecleucel and experienced grade 2 CRS without ICANS. On day +28, he was MRD− by flow and had achieved BCA. Next-generation sequencing (NGS)-MRD was sent and is positive at <1 cell, below the level of quantitation of 1 × 10−6.
Post–CAR T-cell infusion monitoring and consolidative HSCT considerations
Once remission has been obtained after tisagenlecleucel, based upon long-term data from the ELIANA trial (N = 75), about half of patients will have continued remission without interval therapy and possibly be cured at 5 years; however, the other half will relapse. Given limited salvage options for CAR T-cell relapse23,60 particularly for LS (Figure 1C), clinicians are faced with a choice: send all patients empirically to HSCT, selectively move to HSCT for high-risk patients only, or reserve HSCT for patients who relapse and get back into remission. In the absence of studies designed to define best outcomes, we will review the first 2 of these options while sharing available data.
Consolidative HSCT for all patients receiving CD19 CAR T cells
For patients receiving CD19-directed CAR T-cell constructs, where long-term remissions are not expected (eg, CD28-based CAR T cells), HSCT is critical. Differing from conclusions of an early study of CD28 CAR T cells in adults where OS with or without HSCT was poor,61 consolidative HSCT usually leads to long-term, durable remission in younger patients.10 The challenge is deciding when to take patients to HSCT after treatments similar to tisagenlecleucel, which have curative potential. A recent meta-analysis addressed this issue,62 but the trials in the analysis included mainly adult patients, used constructs that had not demonstrated efficacy for long-term responses without HSCT, and planned for HSCT rather than randomly assign patients or try to define patients who could avoid HSCT. Two pediatric studies give better insight into this question. Investigators in Seattle looked retrospectively at their outcomes with consolidative HSCT after CAR T cells. In 50 patients potentially eligible for HSCT after achieving and maintaining an MRD− remission beyond day 63 after a 4-1BB CAR T-cell construct with long-term curative potential, HSCT-naïve patients receiving a post–CAR consolidative HSCT had a trend toward improved leukemia-free survival (LFS) (P = .09), whereas those with a history of prior HSCT had no advantage in LFS (P = .45). No advantage was noted in OS, as many patients were salvaged with additional therapy. Patients who had loss of BCA/CAR T-cell disappearance by day 63 and received a consolidative first HSCT showed a clear improvement in LFS (P = .01).63 A study from China of children receiving either CD19- or CD22-directed CAR T-cell therapy showed a 1-year EFS of 73% with planned HSCT,64 which compares to approximately 50% EFS in the ELIANA study4 and other real-world27 or collaborative reports.20 These studies suggest an advantage for HSCT after CAR T cells only in HSCT-naïve patients, especially if they lose BCA early, but the experiences are small, single-center, and nonrandomized.
Consolidative HSCT for high-risk patients
Given the identification of risk factors affecting outcomes (Table 1) and poor salvage rates after relapse,23 we are now better informed about patients at high risk of poor outcomes. But there is a sizable portion of patients in poor risk groups who can be cured with tisagenlecleucel alone, so post–CAR T-cell risk factors to better predict patients who will fail are important. To date, there have been only 2 factors post–CAR T-cell therapy that reliably predict relapse: loss of BCA and detection of NGS-MRD.
BCA is a functional screen for active CAR T cells, and loss of BCA before 6 months is associated with very high rates of relapse.49 The measure is suboptimal, however, as patients who relapse with CD19-negative ALL can relapse despite CAR T-cell persistence.23 Because CD19-negative relapse occurs frequently, measuring for BCA alone is inadequate. In addition, the power of BCA to predict outcome fades with time, with 1 study showing that loss of BCA in the first 6 months led to an EFS rate lower than 20%, whereas patients who lost BCA at 1 year had an EFS rate of 75%.36
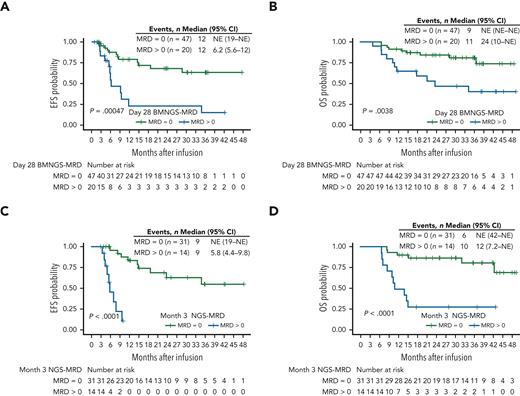
NGS-MRD can potentially overcome the limitations of following BCA alone by tracking the leukemia genomic clone directly, detecting both CD19+ and CD19− disease. Using NGS of blast-specific immunoglobulin heavy chain (IgH) rearrangements to test for MRD after CAR T-cell therapy, considering any level of disease detected positive, multivariate analysis at day 28 showed an independent association of NGS-MRD with relapse (HR, 4.87; 95% confidence interval, 2.18-10.8; P < .001), which increased at 3 months to a HR of 12 (95% confidence interval, 2.87-50; P < .001; Figure 2).36 Although some may consider NGS-MRD detection of blasts simply a more sensitive measure of relapse, it is not part of current definitions of relapse.65 Detection at day 28 after CAR T-cell therapy can be part of an ongoing response and should be verified (Figure 2A). By 3 months, however, any presence of NGS-MRD is highly predictive of relapse (Figure 2C). Another study showed that detection of disease by IG/T-cell receptor RQ-polymerase chain reaction (PCR)–based MRD at day +28 after CAR T-cell therapy is also highly predictive22 and can be used in situations where NGS-MRD is not available.
EFS and OS in patients in CR/CRi following tisagenlecleucel by Kaplan-Meier analyses with log-rank test P values. (A-B) EFS (A) and OS (B) of responding patients based on detection at 28 days after CAR infusion of BM NGS-MRD at any level (blue lines) compared with patients with BM NGS-MRD = 0 (green lines). (C-D) EFS (C) and OS (D) of responding patients based on detection at 3 months after CAR infusion of BM NGS-MRD at any level (blue lines) compared with patients with BM NGS-MRD = 0 (green lines). CRi, complete remission with incomplete count recovery. Reproduced with permission from CCC Marketplace, license number 1257504-1, from Pulsipher et al.36
EFS and OS in patients in CR/CRi following tisagenlecleucel by Kaplan-Meier analyses with log-rank test P values. (A-B) EFS (A) and OS (B) of responding patients based on detection at 28 days after CAR infusion of BM NGS-MRD at any level (blue lines) compared with patients with BM NGS-MRD = 0 (green lines). (C-D) EFS (C) and OS (D) of responding patients based on detection at 3 months after CAR infusion of BM NGS-MRD at any level (blue lines) compared with patients with BM NGS-MRD = 0 (green lines). CRi, complete remission with incomplete count recovery. Reproduced with permission from CCC Marketplace, license number 1257504-1, from Pulsipher et al.36
Unfortunately, when using NGS-MRD, about 10% of patients with B-ALL will not have informative IgH clones, as used in the aforementioned study. Use of T-cell receptor clones may improve that number to >95%, but these clones vary in specificity.66 Accessibility to commercial NGS-MRD testing is another substantial limitation. Consultation with experts in this assay may be needed for suspicious results. Key factors strengthening the predictive power of this test include positivity in multiple clones and a clear pattern of increase in clonal numbers with serial testing.
Given the need to prospectively study these critical biomarkers, the upcoming CAR-CURE trial (ClinicalTrials.gov NCT05621291) will test the hypothesis that systematic surveillance of BCA and NGS-MRD measurements after CD19 CAR T cells can identify those at highest risk for relapse after CAR T-cell infusion and route them to HSCT before relapse.67
Approaches to patients treated with CAR T cells for relapse after HSCT
Given the risks and complications associated with second HSCT,68 post–CAR T-cell consolidative second HSCTs cannot be routinely recommended. Selecting a more persistent CAR T cell is recommended for these very high-risk patients, for whom curative options are limited. Data reporting on outcomes for second HSCT after CAR T-cell–induced remission are based on small cohorts and do not show the same degree of benefit of HSCT compared with transplant-naïve patients.69,70 Therefore, for these patients, experimental CAR T-cell approaches aimed at improving cure as a single therapy or strategies aimed at preventing relapse should be encouraged, with second HSCT reserved only for those clearly at imminent risk of relapse.
Case (continued): repeat IgH NGS-MRD remains low-level positive at day +60 with concurrent loss of BCA as the absolute CD19+ count rises to 75/μL. The HSCT team plans to move forward with the transplant as soon as possible, and a fully matched unrelated donor has been identified.
Approach after identification of patients at high risk for relapse after CAR T-cell infusion
Bridging high-risk patients to HSCT
With early loss of BCA and/or persistent or rising NGS-MRD, relapse may be imminent.36 Because of the high rates of survival when patients are NGS-MRD− before HSCT,71 initiating therapy to induce or maintain NGS-MRD negativity is often considered, particularly when awaiting donor availability. Given the chemotherapy refractory nature of the disease in patients who received CAR T cells and because NGS-MRD cannot elucidate the leukemic immunophenotype, bridging options are both limited and empiric. Blinatumomab may be particularly effective with low disease burden if CD19+ is retained,72,73 but as antigen expression is not known with NGS-MRD, efficacy may be limited if the disease is CD19−. Inotuzumab ozogamicin, a CD22-targeted conjugated antibody, is a consideration but particularly problematic in the peri-HSCT setting, given concerns for veno-occlusive disease/sinusoidal obstructive syndrome.46,74 Nonimmunotherapeutic and antigen agnostic chemotherapy could also be used, though with caution given the general chemotherapy refractory nature of disease in patients receiving CAR T cells.
Proceeding to HSCT in patients who are RQ-PCR+ or NGS-MRD+ and flow MRD–negative may not be ideal, but it may be reasonable, as HSCT may be the only curative option and treatment of the residual disease may not be feasible or effective. When pre-HSCT flow cytometry is <0.01% or PCR is <10−4, survival is clearly improved.75 However, in patients who are NGS-MRD+ but flow cytometry–negative, developing some level of acute graft-versus-host disease and undergoing a myeloablative total body irradiation–based conditioning may be necessary to optimize chances of long-term survival.71
CAR T-cell reinfusions for relapse prevention
The role of CAR T-cell reinfusion as a relapse prevention strategy for patients with early loss of BCA has not been firmly established, and patients losing CAR T cells before 6 months rarely have long-term responses. Several groups have reported limited success with reinfusion.32,76,77 However, investigators at Children’s Hospital of Philadelphia, using an approach of early preemptive reinfusions, have reported greater success.78 Among 63 patients reinfused for peripheral B-cell recovery (n = 38) or hematogones in the bone marrow (n = 25), 33 (52%) established or maintained BCA 28 days after reinfusion. Of those, 16 (48%) remained in remission without further therapy at a median of 39 months from reinfusion.78 The addition of checkpoint inhibitors after infusion has been suggested,79 but data showing a clear benefit is lacking.
Case (continued): because the identified unrelated donor had been confirmed, the patient started his preparative regimen for HSCT within 3 weeks with no interval therapy. He developed grade II acute graft-versus-host disease and no chronic graft-versus-host disease, and at 2 years post-HSCT, he remains in an ongoing remission.
Conclusions
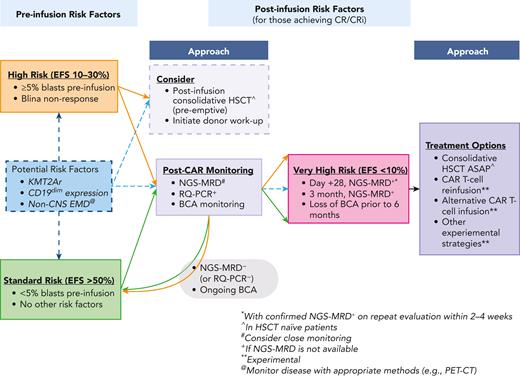
Although CD19 CAR T cells have transformed the treatment of pediatric r/r B-ALL, applying risk factors associated with the success or failure of this therapy to optimize outcomes is a crucial next step. Although we use a single case to illustrate these important considerations, unique populations (eg, infants, those with early CNS relapse, etc) warrant unique considerations, which are outlined in Table 3. Given the potential for long-term cure with CD19 CAR T cells, avoiding allogeneic HSCT and its associated short- and long-term toxicities is greatly desired. However, given that treatment outcomes of relapse after CD19 CAR T cells are dismal,23 it is critical to define the best approaches to prevent relapse in patients at highest risk. In patients for whom consolidative HSCT following CAR T cells is not an option (eg, patient/parent preference or HSCT-limiting toxicities), developing better relapse-prevention strategies is critical. Treatment of pediatric B-ALL has always been built upon risk stratification, and based on current data, we have included our personalized approach to risk stratification of this therapy (Figure 3). We recommend prospective studies to validate and refine approaches aimed at improving outcomes following CD19 CAR T cells in pediatric B-ALL.
Approach to peri-CAR T-cell risk stratification with monitoring and treatment options. This figure highlights various preinfusion risk factors that may affect long-term EFS, remission durability, and/or OS; and how these factors are further modulated by post–CAR T-cell outcomes, based primarily on NGS-MRD status and loss of BCA. Refer to Table 1 for specific information on risk factors. ASAP, as soon as possible; Blina, blinatumomab; CRi, complete remission with incomplete count recovery.
Approach to peri-CAR T-cell risk stratification with monitoring and treatment options. This figure highlights various preinfusion risk factors that may affect long-term EFS, remission durability, and/or OS; and how these factors are further modulated by post–CAR T-cell outcomes, based primarily on NGS-MRD status and loss of BCA. Refer to Table 1 for specific information on risk factors. ASAP, as soon as possible; Blina, blinatumomab; CRi, complete remission with incomplete count recovery.
Acknowledgments
Figure 3 was created with Biorender.com. The authors acknowledge Elizabeth Holland (National Institutes of Health [NIH]) for her support in developing Figure 3.
R.M.M. is supported by NIH, National Cancer Institute (NCI) grant K-12-CA-076931 and the Alex’s Lemonade Stand Foundation. M.A.P. is supported by NIH, National Institute of Allergy and Infectious Diseases grant 1U01AI126612-01A1, NIH, NCI grant P30CA040214, and the St. Baldrick’s Johnny Chrisstopher Fund. This work was supported in part by the Intramural Research Program, Center of Cancer Research, NCI and NIH Clinical Center, and National Institutes of Health (ZIA BC 011823, N.N.S.).
The content of this publication does not necessarily reflect the views of policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the US government.
Authorship
Contribution: M.A.P. and N.N.S. conceived the proposed idea; and all authors contributed to outlining, writing, and editing the manuscript and approved the final version of the manuscript.
Conflict-of-interest disclosure: M.A.P. has participated in advisory boards for Novartis, Gentibio, Bluebird, Vertex, Medexus, Mesoblast, and Equillium. He has given educational talks for Novartis and Adaptive. He receives study support from Adaptive and Miltenyi. N.N.S. received royalties from Syncopation Life Sciences and has participated in advisory boards for Sobi and VOR. R.M.M. declares no competing financial interests.
Correspondence: Michael A. Pulsipher, Huntsman Cancer Institute, 2000 Circle of Hope Dr Rm 3515, Salt Lake City, UT 84112; e-mail: michael.pulsipher@hci.utah.edu.
References
Author notes
∗R.M.M. and N.N.S. contributed equally to this study.


![Impact of previous blinatumomab treatment and disease burden on EFS following CD19 CAR T cells and OS following relapse. EFS, defined as the time from CD19 CAR T-cell infusion to one of the following events: no response, relapse, or death. (A) EFS stratified by blinatumomab-naïve patients (no blina—teal) vs blinatumomab-exposed patients who achieved a CR to blinatumomab (blina-CR—blue) vs blinatumomab-exposed patients who did not achieve a CR to blinatumomab (blina-no CR—red). P values for EFS curves: .59 (no blina vs blina-CR); .01 (blina-CR vs blina-no CR); .001 (no blina vs blina-no CR). (B) EFS stratified by high disease burden (≥5% bone marrow blasts—blue [high]) vs low disease burden (<5% bone marrow blasts—red (low). (C) OS following relapse, stratified by relapse immunophenotype. Median OS for CD19+ relapse was 18.9 months (95% CI, 11.2-27.0 months). Median OS for CD19− relapse was 9.7 months (95% CI, 6.9-15.9 months). Median OS for LS was 3.7 months (95% CI, 1.2-7.0 months). Red: CD19+; blue: CD19−; green: LS. CI, confidence interval; LS, lineage switch. (A-B) Reproduced with permission from Wolters Kluwer Health, license number 5363041439390, from Myers et al20; (C) reproduced with permission from Elsevier, license number 5363050768789, from Lamble et al.23](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/141/11/10.1182_blood.2022016937/4/m_blood_bld-2022-016937-c-gr1.jpeg?Expires=1765928269&Signature=ai8OFC~4hojIMa1iIYf-MBJp5J-wALgTkzieX1hEB7l-ZOwFSMLlHMifSPOczqCCWpg6fa0idJDuVT4epo9cKwad7U0SgLnGj3iYQlsKrXs5NcA582eTmE5vwqT8NgYyyKAk4vL6A4MOor7-NtvKVbPQxy1-qVTyHPxJ3vOHneK4A-85Q8lNg-K~HY4cmCv~uZtsA7IsOWEbTcmOGCRIuNFbeB8LDrGuIKAoa8--mE~~-bcsoAmYwuS6i3m~GHueeDtjE6Pes-4NgMrZki7-ztNd09pQnPFc4zzvwMzpXPPjB2-9NSKRxNnhJjtPzxPHz2qSAyiLg2JN0JkUWycTcg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

![Impact of previous blinatumomab treatment and disease burden on EFS following CD19 CAR T cells and OS following relapse. EFS, defined as the time from CD19 CAR T-cell infusion to one of the following events: no response, relapse, or death. (A) EFS stratified by blinatumomab-naïve patients (no blina—teal) vs blinatumomab-exposed patients who achieved a CR to blinatumomab (blina-CR—blue) vs blinatumomab-exposed patients who did not achieve a CR to blinatumomab (blina-no CR—red). P values for EFS curves: .59 (no blina vs blina-CR); .01 (blina-CR vs blina-no CR); .001 (no blina vs blina-no CR). (B) EFS stratified by high disease burden (≥5% bone marrow blasts—blue [high]) vs low disease burden (<5% bone marrow blasts—red (low). (C) OS following relapse, stratified by relapse immunophenotype. Median OS for CD19+ relapse was 18.9 months (95% CI, 11.2-27.0 months). Median OS for CD19− relapse was 9.7 months (95% CI, 6.9-15.9 months). Median OS for LS was 3.7 months (95% CI, 1.2-7.0 months). Red: CD19+; blue: CD19−; green: LS. CI, confidence interval; LS, lineage switch. (A-B) Reproduced with permission from Wolters Kluwer Health, license number 5363041439390, from Myers et al20; (C) reproduced with permission from Elsevier, license number 5363050768789, from Lamble et al.23](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/141/11/10.1182_blood.2022016937/4/m_blood_bld-2022-016937-c-gr1.jpeg?Expires=1766018641&Signature=lLwRgonnQISPOwa3wEbX8~kGPaHO0LYAGFY~M2pRKLhOD87Kuj8hIROsoqs1Z~aVZpuMzp24GdLonwbLKRhgnTt0xQsOcb73O3I1V6uNvFtQWezM8e3QoTKSbCOkatknCjQz7SaxD1JziaXQterivW6BJg6GjOwB3i0-qo6tyBQqJEOdUdOB2n~S6Sx-0A379sldXT97ALIArnBomJw5W7fhX1sL-Uo2nv5pjmb4HcE76SjH8Ik60GNbZFb7gLUYKisPt42mOALX-UOHCkkCOt~DcTdkEKROMju4wZ9NAkjNHHhR6kMjwvck9371pKYPESYTerNfDIeMZHVFUF4fGw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)