Abstract
Background Venetoclax (Ven) in combination with azacitidine (Aza) has improved the treatment outcome in acute myeloid leukemia (AML) patients (pts) not benefitting from intensive chemotherapy. Nevertheless, treatment failure remains a challenge, and predictive markers are needed, particularly for relapsed or refractory (R/R) AML. While certain genetic changes may correlate with treatment outcomes, mutations cannot precisely predict response to Ven-Aza. In the VenEx trial we used ex vivo Ven-sensitivity testing to predict therapy response and overall survival.
Methods VenEx is a multicenter, open-label phase II trial conducted by the Finnish AML Group (NCT04267081). The trial recruited 104 pts: 48 nonfit de novo and 56 R/R or secondary AML (sAML) pts. Ex vivo Ven-sensitivity testing was performed at screening for all pts. The primary endpoint was the treatment response in ex vivo Ven-sensitive pts during the first three therapy cycles and the key secondary endpoints were the correlation of sensitivity with responses and survival.
For drug sensitivity testing, bone marrow mononuclear cells were plated in conditioned medium (CM; HS-5 cell line derived media) to drug plates within 26 hours after sampling. After 48 h, blast viability (CD34pos, CD117pos or SSClowCD45low) was analyzed by flow cytometry using Annexin V and 7-AAD. Ven-sensitivity was measured in 7 different concentrations, and a drug sensitivity score (DSS) derived from the area under the dose response curve calculations indicated efficacy (Yadav et al., 2014).
In the first stage of the trial, all 39 pts received Ven-Aza combination in 28-day cycles regardless of drug testing results (Ven: 400 mg once daily for 28 days, shortened to 21 days in responding pts; Aza: 75 mg/m2 on days 1-7). The first stage demonstrated that experimental conditions had considerable influence on the predictive value of Ven-sensitivity testing. We observed the best in vivo/ex vivo correlation using CM coupled with a blast-specific flow cytometry assay and defined the optimal cut-off value (DSS 10.7) for sensitivity (Kuusanmäki et al., ASH 2021).
The second stage of the trial recruited 65 pts. Based on the results from the first stage, all de novo AML patients received Ven-Aza combination, whereas the pts with R/R or sAML were selected for therapy using Ven-sensitivity testing. Ex vivo resistant pts (DSS < 10.7) were excluded from study therapy (N = 12). Overall survival (OS) was estimated by the Kaplan-Meier method. The data-cutoff was June 27, 2022; pts alive at the cutoff date were censored for the OS analysis.
Results Drug sensitivity testing was technically successful in 102/104 pts and all results were communicated to the site within 3 days from sampling. Using a DSS threshold of 10.7, 86% (N = 64/74) of ex vivo Ven-sensitive pts achieved a CR/CRi/MLFS response, and 73% (N = 54/74) achieved a CR/CRi response. In the R/R or sAML cohort, 82% (n = 28/34) of ex vivo Ven-sensitive pts achieved a CR/CRi/MLFS response and, and 62% (N = 21/34) achieved a CR/CRi response.
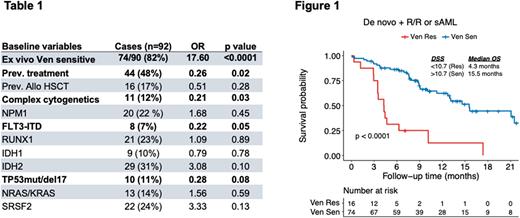
Based on univariate analysis, complex karyotype (OR 0.21, p = .026), previous MDS/AML treatment (OR 0.26, p = .019), FLT3-ITD mutation (OR 0.22, p = .05), and TP53 mutation or 17p deletion (OR 0.28, p = .08) were associated with poor response rates, whereas ex vivo Ven-sensitivity was the only statistically significant predictor for good treatment response (CR/CRi/MLFS; OR 17.6, p < .0001; Table 1).
Median OS for ex vivo Ven-resistant pts receiving Ven was 4.3 months (95% CI 2.1-5.0, p < 0.001) and for ex vivo Ven-sensitive pts, it was 15.5 months (95% CI 12.5-18.6, p < 0.001; Figure 1). For ex vivo Ven-resistant R/R or sAML pts receiving Ven (n=8), median OS was 3.5 months (95% CI 2.6-4.3, p < 0.001) and for ex vivo Ven-sensitive pts (n=34), it was 9.0 months (95% CI 8.7-9.3, p < 0.001). Median OS for ex vivo Ven-resistant pts who received Ven-Aza was similar to those pts who were excluded from study therapy (3.5 vs. 2.2 months).
Conclusions Ex vivo drug sensitivity testing was feasible for AML patients in a clinical trial context. Leukemic blast-specific ex vivo Ven-sensitivity showed a high correlation with treatment responses, and univariate analysis showed Ven-sensitivity to be the best predictor for treatment response. Further, ex vivo Ven-sensitivity predicted longer OS - and importantly, this was also observed in R/R/sAML pts, suggesting the method's usefulness in selecting pts for Ven-Aza therapy.
Disclosures
Partanen:AbbVie: Membership on an entity's Board of Directors or advisory committees; Behring: Membership on an entity's Board of Directors or advisory committees. Porkka:Incyte: Research Funding; Celgene/Bristol-Myers Squibb: Research Funding; Pfizer: Honoraria; Novartis: Honoraria; Pfizer: Research Funding; Incyte: Honoraria; Bristol-Myers Squibb: Honoraria; Astellas: Honoraria; AbbVie: Honoraria; Novartis: Research Funding. Heckman:IMI2 projects HARMONY and HARMONY PLUS: Research Funding; WntResearch: Research Funding; Oncopeptides: Research Funding; Orion: Research Funding; Kronos Bio: Research Funding; Novartis: Research Funding; Celgene: Research Funding. Ettala:Takeda: Consultancy; Amgen: Membership on an entity's Board of Directors or advisory committees; Sanofi: Membership on an entity's Board of Directors or advisory committees; Novartis: Membership on an entity's Board of Directors or advisory committees; Pfizer: Membership on an entity's Board of Directors or advisory committees. Rimpiläinen:Novartis: Membership on an entity's Board of Directors or advisory committees; Pfizer: Membership on an entity's Board of Directors or advisory committees; AbbVie: Membership on an entity's Board of Directors or advisory committees; Bristol-Myers Squibb: Membership on an entity's Board of Directors or advisory committees. Siitonen:AbbVie: Consultancy, Membership on an entity's Board of Directors or advisory committees; Bristol Myers Squibb: Consultancy, Membership on an entity's Board of Directors or advisory committees; Janssen-Cilag: Membership on an entity's Board of Directors or advisory committees; Celgene: Membership on an entity's Board of Directors or advisory committees; Pfizer: Membership on an entity's Board of Directors or advisory committees. Pyörälä:Pfizer: Membership on an entity's Board of Directors or advisory committees; AbbVie: Membership on an entity's Board of Directors or advisory committees; Novartis: Membership on an entity's Board of Directors or advisory committees; Bristol-Myers Squibb: Membership on an entity's Board of Directors or advisory committees. Kuusanmäki:Faron: Consultancy; AbbVie: Research Funding. Kontro:Astellas Pharma: Consultancy; AbbVie: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Bristol-Myers Squibb: Consultancy, Membership on an entity's Board of Directors or advisory committees; Faron Pharmaceuticals: Consultancy, Membership on an entity's Board of Directors or advisory committees; Novartis: Membership on an entity's Board of Directors or advisory committees; Pfizer: Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.