Abstract
Patients with hematologic malignancies and recipients of hematopoietic cell transplantation (HCT) are more likely to experience severe coronavirus disease 2019 (COVID-19) and have a higher risk of morbidity and mortality after infection with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Compared with the general population, these patients have suboptimal humoral responses to COVID-19 vaccines and subsequently increased risk for breakthrough infections, underscoring the need for additional therapies, including pre- and postexposure prophylaxis, to attenuate clinical progression to severe COVID-19. Therapies for COVID-19 are mostly available for adults and in the inpatient and outpatient settings. Selection and administration of the best treatment options are based on host factors; virus factors, including circulating SARS-CoV-2 variants; and therapeutic considerations, including the clinical efficacy, availability, and practicality of treatment and its associated side effects, including drug-drug interactions. In this paper, we discuss how we approach managing COVID-19 in patients with hematologic malignancies and recipients of HCT and cell therapy.
Introduction
An outbreak of a new respiratory illness called coronavirus disease 2019 (COVID-19) originating from Wuhan, China, was identified in December 2019 and is caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). The World Health Organization declared COVID-19 a public health concern on January 30, 2020, and subsequently declared the novel coronavirus outbreak a global pandemic on March 11, 2020.1
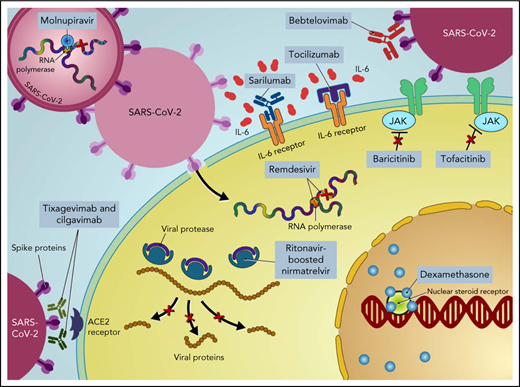
Most infections caused by SARS-CoV-2 are not severe. However, the spectrum of infection severity depends on the underlying host risk factors and virulence of SARS-CoV-2 variants. Severe illness is predominantly associated with increased age (>65 years) and underlying medical comorbidities, such as diabetes mellitus, hypertension, and chronic lung disease.2 Patients with cancer, particularly those with hematologic malignancies, and hematopoietic cell transplant (HCT) recipients are more likely to have severe disease, with increased morbidity and mortality.3-6 Furthermore, compared with the general population, cancer patients and HCT and cellular therapy recipients have suboptimal immune responses following COVID-19 vaccination,7-10 underscoring the need for additional therapies to prevent severe outcomes in these patients (ie, pre- and postexposure prophylaxis).11,12 Moreover, COVID-19 is primarily driven by the replication of SARS-CoV-2 early in the disease process.13 Thereafter, disease severity is driven by host immune dysregulation, resulting in hyperinflammation, organ damage, and an increased risk of thrombosis.14 Therefore, incorporating different therapeutic agents at different disease stages is important to prevent disease progression, morbidity, and mortality (Figure 1).
How I treat
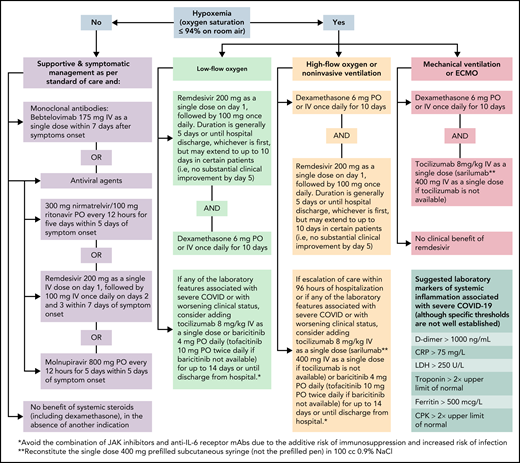
To illustrate how we approach COVID-19 in patients with hematologic malignancies and recipients of HCT and cell therapy, we present different clinical scenarios describing common encounters and practices across cancer centers. Concepts and approaches to assessing, preventing, and treating COVID-19 in these vulnerable patients are presented as clinical vignettes with summary points that should remain foundational in the care of these patients, even as new therapeutic and preventative strategies become available. However, we acknowledge that this discussion has several limitations. First, understanding of COVID-19 is evolving, and the data provided in this “How I Treat” article are based on the available literature and experience at the time of this publication (Algorithm). To address this limitation, we have provided hyperlinks to online resources for the most up-to-date information on treatment guidelines and therapies. Second, our recommendations are largely based on the results of randomized controlled trials (RCTs) conducted mostly in patients who are at high risk for severe COVID-19 due to comorbidities as limited numbers of cancer or cellular therapy recipients are enrolled in such gold-standard clinical trials. Therefore, most guidance is based on expert opinion, clinical experience, and currently available data and resources.
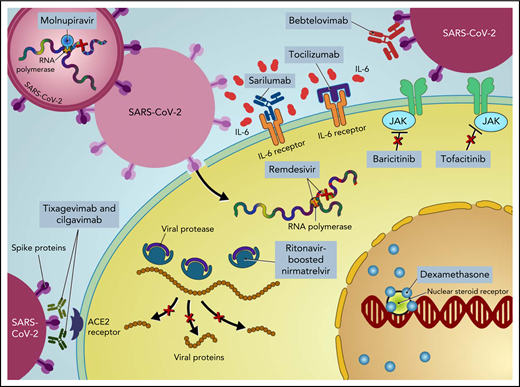
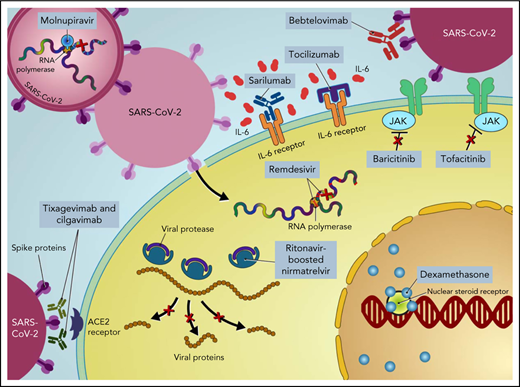
Treatment of COVID-19 in patients with hematologic malignancies and recipients of cellular therapies. CPK, creatine phosphokinase; CRP, C-reactive protein; ECMO, extracorporeal membrane oxygenation; LDH, lactate dehydrogenase; PO, by mouth.
Treatment of COVID-19 in patients with hematologic malignancies and recipients of cellular therapies. CPK, creatine phosphokinase; CRP, C-reactive protein; ECMO, extracorporeal membrane oxygenation; LDH, lactate dehydrogenase; PO, by mouth.
Prevention of SARS-CoV-2 infection
Case 1
A 42-year-old man with adverse-risk acute myeloid leukemia (AML) who was in complete remission after receiving 1 cycle each of induction and consolidation chemotherapy underwent matched related donor allogeneic HCT (allo-HCT) with a myeloablative conditioning regimen. He was discharged on day 32 following allo-HCT with standard antimicrobial prophylaxis. He was concerned during his first clinic visit about his risk of contracting SARS-CoV-2. He was afebrile and denied any respiratory or gastrointestinal symptoms or any recent exposure to SARS-CoV-2. What type of preexposure prophylaxis would you recommend?
Most studies suggest that patients with immunocompromised conditions such as cancer will experience a suboptimal immune response to any COVID-19 vaccine compared with the general population.15-17 For example, limited immunogenicity in patients with solid and hematologic malignancies has been shown after 1 dose of the BNT162b2 vaccine. Specifically, lower concentrations of positive anti-S immunoglobulin G (IgG) titers were measured at approximately 21 days following a single vaccine inoculum compared with concentrations in healthy control samples (38% in patients with hematologic malignancies vs 94% in healthy control samples).18 Furthermore, cancer was associated with a higher risk of breakthrough infections (odds ratio [OR], 1.1) and severe outcomes (OR, 1.3).19 This suboptimal immune response to vaccination is driven by the immunosuppressive state of the cancer type as well as acquired immunodeficiency from anticancer treatments, particularly B-cell–depleted therapies and the conditioning regimen for cellular therapies.20,21
Despite being associated with poor antibody induction in immunocompromised patients, vaccination remains the first-line strategy to prevent SARS-CoV-2 infection or at least COVID-19–related complications such as hospitalization or mortality. Notwithstanding poor vaccine-induced humoral immunity, vaccination may provide long-term T-cell–mediated immunity, regardless of antibody titers and even in patients receiving anti–B-cell–directed agents,22 decreasing the risk of breakthrough infections and the rates of severe disease and hospitalization.23,24 Additional doses of COVID-19 vaccines may enhance immunogenicity, particularly in patients with lower antibody titers following the initial vaccine inoculum.18,25 Compared with neutralizing activity against the ancestral SARS-CoV-2 strain following 2 doses of mRNA or adenovirus vector COVID-19 vaccines, the activity of neutralizing antibodies against B.1.1.7 (α), B.1.351 (β), and B.1.617.2 (δ) variants is diminished.26 Nevertheless, decreased neutralizing antibodies against variants of concern (VOC), even the B.1.1.529 (ο) variant, can be enhanced with a third full dose of the COVID-19 vaccine.27,28 Interestingly, the mRNA-1273 vaccine seems to be the most immunogenic in patients with cancer, followed by BNT162b2 and Ad26.COV2.S, as measured by neutralizing antibody response.25
On the basis of the results of the phase 3 double-blind randomized clinical trial PROVENT, the US Food and Drug Administration (FDA) issued an emergency use authorization (EUA) on December 8, 2021, for tixagevimab and cilgavimab as preexposure prophylaxis of COVID-19 in patients with weakened immune systems who have not been recently exposed to an individual with SARS-CoV-2 infection.29 Although a 77% reduction in the incidence of infection occurred in the treatment arm (95% confidence interval[CI], 46-90%; P < .001), tixagevimab and cilgavimab should only be used in individuals who cannot receive or are anticipated to experience a suboptimal or inadequate response to a COVID-19 vaccination. Of note, the PROVENT trial included a small number of immunocompromised individuals. Furthermore, the emergence of VOC with reduced susceptibility to tixagevimab and cilgavimab may increase the risk of treatment failure. Therefore, it is imperative to consider the regional prevalence of circulating VOC when using such therapies.30
In a patient with AML <3 months after allo-HCT, we elected to administer 300 mg each of tixagevimab and cilgavimab, with the caveat that the higher dose is only based on pharmacokinetic and pharmacodynamic modeling, as this higher dose has in vivo activity against the ο subvariants.31 Relative to comparators, ο BA.2 retains near full susceptibility to tixagevimab and cilgavimab, but ο BA.1 and BA.1.1 reduces susceptibility by 12- to 424-fold, respectively.31
Currently, the phase 3 clinical trial TACKLE is evaluating the efficacy and safety of higher doses of tixagevimab and cilgavimab for the prevention of severe COVID-19 in nonhospitalized patients with mild to moderate COVID-19 (ClinicalTrials.gov NCT04723394). On the basis of ASTCT (American Society for Transplantation and Cellular Therapy) COVID-19 guidelines,32 a full COVID-19 mRNA vaccination series was recommended to our patients, starting ≥3 months after allo-HCT.33
Case summary points:
- 1.
Patients with underlying malignancies and recipients of HCT are at increased risk for severe COVID-19.
- 2.
Given the induction of attenuated antibodies following SARS-CoV-2 vaccination and vaccine-associated antibody neutralization against different VOC, immunocompromised patients, including patients with hematologic malignancies and HCT recipients, should receive a third SARS-CoV-2 mRNA vaccination and booster doses, as well as undergo revaccination with primary series and boosters after HCT or other cellular therapies, as indicated.
- 3.
Preexposure prophylaxis with tixagevimab and cilgavimab is currently recommended in patients with hematologic malignancies and those receiving HCT or cellular therapies. However, changes in the epidemiology of circulating VOC may affect this recommendation in the future.
- 4.
In individuals who have received a COVID-19 vaccine, tixagevimab and cilgavimab should be administered at ≥2 weeks after vaccination.
Management of COVID-19 in nonhospitalized patients
Case 2
A 56-year-old man with diffuse large B-cell lymphoma who completed 4 cycles of R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone) 2 months previously presented to the clinic with a 1-day onset of rhinorrhea, cough, fever, and myalgia. His oxygen saturation on room air was 96%, and his temperature was 101°F. Nasopharyngeal SARS-CoV-2 reverse transcription-polymerase chain reaction (RT-PCR) testing was positive, and a respiratory viral panel was negative for other viruses. His chest radiograph showed no infiltrates. He completed his COVID-19 vaccination series >4 months previously and had positive antispike (S) IgG titers 21 days after his first booster shot. He was concerned about his COVID-19 diagnosis and worried about progression to severe infection, with the potential risk of hospitalization and poor outcome. What type of postexposure prophylaxis or treatment would you recommend in patients who do not require hospitalization at the time of diagnosis?
Patients with cancer who contract COVID-19 have a significantly higher risk of adverse outcomes,3,4 especially those with hematologic malignancies receiving cytotoxic therapy.4 Furthermore, patients with B-lymphoid malignancies who received anticancer therapy within 12 months of COVID-19 diagnosis may experience increased COVID-19-related complications such as increased rates of hospitalization and intensive care unit use.34 Despite the added risk of severe disease, management options for COVID-19 in cancer patients are similar to those for other high-risk patients with different comorbidities (Table 1).
Anti-SARS-CoV-2 monoclonal antibodies (mAbs)
In the United States, EUA has been granted by the FDA for several anti–SARS-CoV-2 mAbs for the treatment and postexposure prevention of COVID-19 in select individuals at high risk for severe COVID-19. Specifically, anti–SARS-CoV-2 mAbs have been authorized for patients who have symptomatic, mild to moderate COVID-19 that does not require supplemental oxygen. However, the emergence of VOC such as ο B.1.1.529 has resulted in casirivimab–imdevimab, bamlanivimab–etesevimab, and sotrovimab no longer being recommended where the ο BA.2 subvariant is prevalent, given their reduced in vitro activity. Bebtelovimab remains active against the ο subvariants BA.1 and BA.2,35,36 at least in vitro, and is currently administered within 7 days after symptom onset for the treatment of nonhospitalized high-risk patients with mild to moderate COVID-19.
Antiviral therapy
Ritonavir-boosted nirmatrelvir
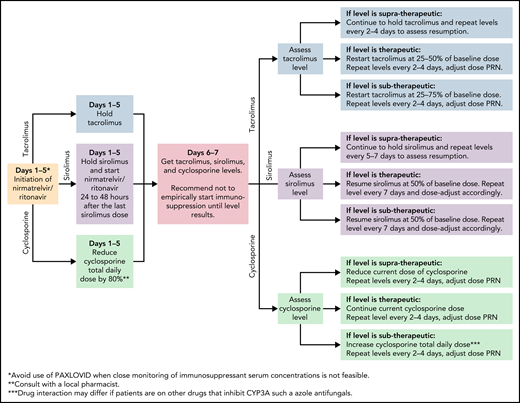
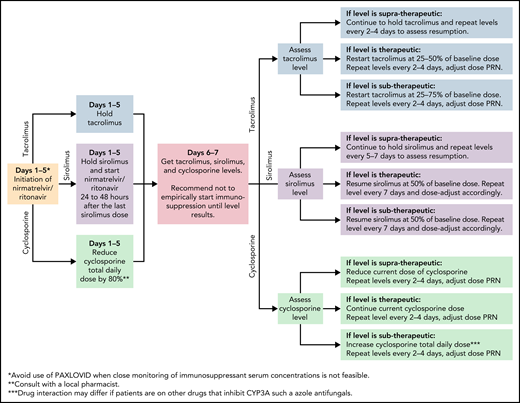
In the EPIC-HR trial, ritonavir-boosted nirmatrelvir reduced the rate of progression to severe COVID-19 by 89% and 88% when administered within 3 and 5 days after symptom onset, respectively, in high-risk, nonhospitalized adults.37 Nirmatrelvir is a protease inhibitor active against the chymotrypsin-like cysteine viral protease MPRO, and ritonavir enhances its pharmacokinetics38 (Figure 1). Ritonavir is a strong cytochrome P450 3A4 inhibitor with significant potential for drug–drug interactions when prescribing ritonavir-boosted nirmatrelvir. Strategies to overcome drug–drug interactions include adjusting the dose or withholding concomitant medications that may interact with ritonavir-boosted nirmatrelvir; performing frequent therapeutic drug level monitoring, particularly for calcineurin inhibitors and mammalian target of rapamycin inhibitors, to avoid supratherapeutic exposure; and using alternative medications when possible (Figure 2). If a potential drug–drug interaction is identified, it will be important to adjust the interacting medications rather than altering the dose of ritonavir-boosted nirmatrelvir. Whenever these above strategies are not possible, it is prudent to consider alternative COVID-19 therapies. Ritonavir-boosted nirmatrelvir dosing needs to be adjusted with renal and hepatic impairments.
Managing immunosuppressants in the setting of nirmatrelvir and ritonavir use. JAK, Janus kinase; PRN, pro re nata (as needed).
Managing immunosuppressants in the setting of nirmatrelvir and ritonavir use. JAK, Janus kinase; PRN, pro re nata (as needed).
Emerging reports describe patients with laboratory-confirmed SARS-CoV-2 infection experiencing a rebound of COVID-19 symptoms 2 to 8 days after completing 5 days of ritonavir-boosted nirmatrelvir.39-41 Despite retesting positive for the virus, these patients clinically improved without additional antiviral-directed therapy. Notably, in the EPIC-HR clinical trial of ritonavir-boosted nirmatrelvir treatment for nonhospitalized adults with COVID-19, 1% to 2% of patients who completed treatment had either a positive SARS-CoV-2 test after testing negative or an increase in the SARS-CoV-2 viral load by PCR.37 At this time, there are no clear signals of baseline or treatment-emergent resistance. Treatment with remdesivir should be considered for immunocompromised patients with “rebound phenomenon” on a case-by-case basis. In addition, the possible transmission was described during this “rebound phenomenon,”39 quarantine for 5 full days after onset of the rebound symptoms, and wearing a face mask for an additional 5 days is recommended.
Remdesivir
Remdesivir is a nucleotide prodrug of an adenosine analog that is incorporated into nascent viral RNA chains, resulting in premature termination42 (Figure 1). Remdesivir has demonstrated in vitro activity against SARS-CoV-2.43 In a double-blind, placebo-controlled RCT in nonhospitalized patients with COVID-19 who are at high risk for disease progression, a 3-day course of IV remdesivir resulted in an 87% relative reduction in the risk of hospitalization or death compared with placebo.44 Of note, vaccinated individuals were excluded from that trial, only 30 (5.3%) patients had a current cancer diagnosis, and the key inclusion criteria were laboratory-confirmed SARS-CoV-2 infection ≤4 days from screening and symptom onset ≤7 days from randomization. Remdesivir has the advantage of resulting in fewer drug–drug interactions while maintaining comparable risk reductions to ritonavir-boosted nirmatrelvir. However, the 3-day IV dosing regimen poses logistical challenges, particularly for outpatient administration.
Molnupiravir
Molnupiravir is a nucleoside analog prodrug of N-hydroxycytidine. Phosphorylated N-hydroxycytidine is incorporated into the viral RNA, leading to an accumulation of deleterious errors in the viral genome and thereby halting the viral replication45-47 (Figure 1). In the phase 3 component of MOVe-OUT placebo-controlled, double-blind RCT, a 5-day course of oral molnupiravir initiated within 5 days of symptom onset reduced the risk of hospitalization and death in nonhospitalized patients with mild to moderate COVID-19 compared with placebo (6.8% [48 of 709] vs 9.7% [68 of 699]; difference, −3.0 percentage points; 95% CI, −5.9 to −0.1).48 Although the trial included patients with ≥1 risk factor for the development of severe COVID-19, only 29 (2%) patients had active cancer, and only unvaccinated patients were eligible. Although it has not been compared head-to-head with other COVID-19 therapies, molnupiravir seems to have lower efficacy than remdesivir and ritonavir-boosted nirmatrelvir. However, molnupiravir does not require dose adjustment for renal or hepatic impairment and has minimal drug−drug interactions, except for reducing the therapeutic effect of cladribine.
For our patient, we elected to treat with ritonavir-boosted nirmatrelvir based on encouraging data from an RCT for nonhospitalized patients with COVID-19. In addition, our patient was receiving other medications with no major concerns for drug−drug interactions. Another reasonable option would have been IV remdesivir, but this could be logistically challenging for daily outpatient administration. Molnupiravir is an alternative option if the preferred agents (ritonavir-boosted nirmatrelvir and remdesivir) are either not available or contraindicated.
Case summary points:
- 1.
Anti−SARS-CoV-2 mAbs have been shown to decrease progression to severe COVID-19, but efficacy varies by VOC. Therefore, knowing the local and regional prevalence of circulating VOC is critical.
- 2.
Antiviral agents are emerging in the treatment of SARS-CoV-2 infection, with variable efficacy. In general, early initiation of antiviral therapy is associated with better outcomes (Table 1).
- 3.
Careful consideration for potential drug−drug interactions is critical before starting antiviral agents, especially ritonavir-boosted nirmatrelvir (Figure 2).
- 4.
Combinations of different antiviral agents for the treatment of COVID-19 are emerging, but no RCTs of their use have been conducted in cancer patients or HCT and cellular therapy recipients.
- 5.
Despite the emergence of different VOC, remdesivir, ritonavir-boosted nirmatrelvir, and molnupiravir seem to have equipotent activity against α, β, γ, Δ, and ο variants.49
Management of COVID-19 in patients requiring hospitalization
Case 3
A 32-year-old woman with relapsed B-cell acute lymphoblastic leukemia/lymphoma received salvage chimeric antigen receptor T-cell therapy and achieved complete remission, according to repeat bone marrow biopsy and aspiration and positron emission tomography–computed tomography done 30 days previously. Her blood counts recovered, except for the absolute lymphocyte count (120 lymphocytes per microliter of blood), with the lymphocyte profile revealing the absence of CD19+CD20+ B cells. She presented to the emergency department with a 1-day history of shortness of breath, congestion, sinus pain, and fever. SARS-CoV-2 RT-PCR testing was positive, and a respiratory viral panel was negative for other viruses. A chest radiograph showed bilateral scattered opacities consistent with multifocal pneumonia. Oxygen saturation on room air was 82%, which improved to 94% on a 5-L nasal cannula. While the emergency room physician was evaluating her, she developed hypotension requiring vasopressors. She was admitted to the intensive care unit and immediately started on dexamethasone and remdesivir. The ferritin concentration was elevated at 3210 ng/mL, and tocilizumab was administered.
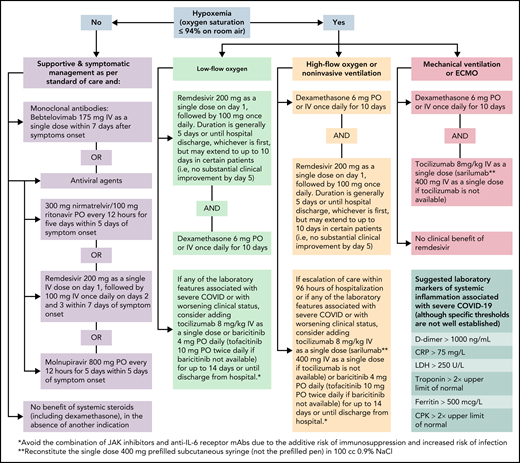
For patients with cancer who are hospitalized with COVID-19 and do not require oxygen, remdesivir should be administered for up to 10 days, as it may shorten the recovery time by a median of 5 days50 (Algorithm). Although other therapies for the management of high-risk outpatients (ie, anti−SARS-CoV-2 mAbs, ritonavir-boosted nirmatrelvir, and molnupiravir) are reasonable alternatives for high-risk hospitalized patients with cancer, remdesivir seems to be a more suitable option, with robust published data on its efficacy and safety in these instances (Table 2). In the absence of another indication, the use of corticosteroids in patients who do not require supplemental oxygenation is not supported.51
For cancer patients who are hospitalized with COVID-19 and are receiving oxygen supplementation, our approach varies based on the degree of oxygen requirement (Algorithm). For patients who require low-flow oxygen support, dexamethasone daily for up to 10 days or until hospital discharge and remdesivir daily for 5 days or until hospital discharge51,52 is recommended. Remdesivir provides the greatest benefit when given early in the course of COVID-19 (ie, within 10 days of symptom onset).44,53 Nevertheless, clinical trials have not shown differences among patients who received remdesivir and those who received standard of care.54,55 Moreover, remdesivir does not show a significant benefit in patients with COVID-19 who require mechanical ventilation.53
For patients who require increasing oxygen supplementation, especially those requiring high-flow oxygenation or noninvasive ventilation, adding a second immunomodulator could provide additional clinical benefits.56 The addition of anti-interleukin 6 (IL-6) receptor mAbs (ie, tocilizumab) or Janus kinase (JAK) inhibitors (ie, baricitinib or tofacitinib) to dexamethasone is reasonable, especially in patients with elevated markers of systemic inflammation57-60 (Table 3). Sarilumab may be used as an alternative to tocilizumab when the latter is unavailable, and tofacitinib may be used in lieu of baricitinib. When a second immunomodulator is contemplated, avoiding the combination of JAK inhibitors and anti−IL-6 receptor mAbs is advised given an increased risk of infection, particularly opportunistic infections and reactivation of latent infections.61
Management of patients with COVID-19 who are mechanically ventilated is similar to the management of hypoxemic respiratory failure due to other causes. Earlier in the pandemic, several retrospective studies reported higher mortality rates with COVID-19−related acute respiratory distress syndrome. Limited data suggest that the clinical features of severe COVID-19 are similar to those of non−COVID-19 with acute respiratory distress syndrome.62
Case summary points:
- 1.
The combination of remdesivir and dexamethasone is the cornerstone of therapy for hospitalized patients with moderate to severe COVID-19.
- 2.
Additional immunosuppressive therapies may be needed to treat the host-derived cytokine storm induced by SARS-CoV-2 infection.
- 3.
Surveillance is critical to ensure that superimposed infections are diagnosed and treated promptly. The need for antimicrobial prophylaxis should be assessed on a case-by-case basis.
Other therapies
Convalescent plasma
In a large multisite RCT, early (ie, within 8 days after symptom onset) outpatient treatment with anti−COVID-19 convalescent plasma decreased the incidence of hospitalization by 54% (absolute risk reduction, 3.4 percentage points; 95% CI, 1.0-5.8; P = .005).63 A smaller clinical trial with early (ie, within 72 hours after the onset of symptoms) high-titer plasma therapy showed a relative risk reduction of 48% for hypoxemia or tachypnea and the risk of progression to severe respiratory disease.64 However, other trials have failed to demonstrate such benefits, probably because of the heterogeneity of the anti−COVID-19 convalescent plasma and the timing of administration.65,66
Given logistical constraints with the collection, preparation, and administration of anti−COVID-19 convalescent plasma, we consider this therapy to be an alternative option for patients with symptomatic COVID-19 infection who are at a higher risk for disease progression if administered early after symptom onset and contain high titers antibodies.
Mesenchymal stem cells
Mesenchymal stem cells (MSCs) are multipotent stem cells with the ability to self-renew and differentiate into various types of tissues. MSCs possess immunomodulatory and antiinflammatory properties, thereby inhibiting SARS-CoV-2 cell-mediated inflammation.67 In addition, they are resistant to SARS-CoV-2 infection, as they lack the angiotensin-converting enzyme 2 receptor used for viral entry into cells68 (Figure 1).
Few studies have investigated different sources of MSCs in the treatment of patients with COVID-19.69 In a double-blind RCT investigating 24 patients who were randomly assigned to receive either umbilical cord MSCs or placebo, no differences in adverse events were noted, and treatment with MSCs was associated with improved survival and time to recovery.70 However, to date, MSCs remain investigational products for the treatment of COVID-19.
SARS-CoV-2–specific T cells
Adoptive T-cell immunotherapy is a promising therapy for viral infections, particularly in immunocompromised patients. Emerging evidence suggests that T cells play an important role in the prevention of COVID-19.71 Transferring off-the-shelf, human leukocyte antigen-matched allogeneic SARS-CoV-2–specific T cells from convalescent individuals has been proven safe for clinical use with no autoreactivity or alloreactivity.72-75 Most importantly, T cells generated from such adoptive immunotherapies are capable of recognizing multiple SARS-CoV-2 variants.76
Natural killer (NK) cells
NK cells play a crucial role in antiviral immune responses.77 Moreover, they can limit tissue fibrosis.78 Individuals with severe COVID-19 have elevated antiinflammatory molecules that impair antiviral defenses by NK cells, leading to poor control of SARS-CoV-2 infection.79-82 NK cells in the blood of patients with COVID-19 express CD39 and upregulate programmed cell death protein 1 receptors and NGKG-2A.83 Given the availability of monoclonal antibodies that target these NK cell molecules, therapies targeting programmed cell death protein 1 (and its ligand, PD-L1), NKG2A, and CD39 are being investigated to boost NK cell antiviral immunity against SARS-CoV-2 infections. However, these therapies should be carefully investigated in severe cases of COVID-19, as highly activated NK cells can worsen lung injury.84
Special considerations
Conclusions
SARS-CoV-2 remains a major threat to patients with hematologic malignancies and recipients of HCT and cellular therapy. SARS-CoV-2 vaccination remains the mainstay strategy to prevent severe COVID-19. However, given decreased vaccine immunogenicity and increased risk for breakthrough infections, additional therapeutic modalities are needed to complement vaccination strategies in patients with hematologic malignancies and recipients of HCT and cell therapy, such as preexposure COVID-19 mAbs (Table 3). Well-designed clinical trials that include this vulnerable patient population are critically needed to identify novel therapies with greater potency and more favorable toxicity profiles to prevent or treat SARS-CoV-2 infection.
Acknowledgments
The authors thank Ann Sutton in Editing Services in the Research Medical Library at The University of Texas MD Anderson Cancer Center for editing the manuscript and David Aten in the Medical Illustration Department at The University of Texas MD Anderson Cancer Center for creating the images.
Authorship
Contribution: F.E.C., J.J.A., and R.F.C. designed the study, reviewed the articles, wrote the manuscript, and gave final approval for the manuscript.
Conflict-of-interest disclosure: R.F.C. has received research funding from Karius, Merck, AiCuris, Viracor-Eurofins, Takeda, Genentech, and Ansun Pharmaceuticals and is a consultant for ADMA Biologics, Janssen, Merck, Molecular Therapeutics, Takeda, Oxford Immunotec, Karius, Shinogi, and Ansun Pharmaceuticals. The remaining authors declare no competing financial interests.
Correspondence: Firas El Chaer, Division of Hematology & Oncology, University of Virginia Comprehensive Cancer Center, West Complex Room 6248, 1300 Jefferson Park Ave, PO Box 800716, Charlottesville, VA 22908; e-mail: fe2gh@virginia.edu.
REFERENCES
Author notes
J.J.A. and R.F.C. are joint senior authors.