Abstract
Immune-mediated thrombocytopenic purpura (iTTP) is a thrombotic microangiopathy characterized by an acquired ADAMTS13 deficiency as a result of the presence of an antibody inhibitor of ADAMTS13 leading to the formation of ultralarge von Willebrand multimers. Treatment of iTTP includes plasma exchange, high-dose glucocorticoids, rituximab, and, more recently, caplacizumab, to prevent the development of exacerbations. There is the risk of both relapse and long-term complications that include neurocognitive deficits and cardiovascular events that occur in patients in remission after recovery from an acute iTTP episode. Data on the risk factors for the development of these complications, the appropriate screening, and treatment are limited due to the paucity of research. This article is a review of the current understanding on the risk factors for exacerbation, relapse, and long-term complications of iTTP and discusses an approach to observing patients with iTTP after hospital discharge and during the long-term follow-up in the outpatient setting.
Introduction
Immune mediated thrombotic thrombocytopenic purpura (iTTP) is an acute thrombotic microangiopathy that is mediated by an acquired, antibody-mediated deficiency of the ADAMTS13 protease. Recent advances in our understanding of iTTP have shifted the focus to improving recovery from an acute iTTP episode and addressing long-term complications of the disease. iTTP is now increasingly recognized as a chronic disease with long-term sequelae. These sequelae include cardiovascular and neurocognitive deficits that may be responsible for the decreased survival that has been reported in patients with a previous diagnosis of iTTP.1,2
Case 1
A 36-year-old White man with a history of 3 prior acute iTTP episodes presented with abdominal pain, nausea for 3 to 4 days, and a headache that began 24 hours earlier. His first iTTP episode occurred at age 29; since then, he has had 2 additional episodes, with the most recent one occurring 3 years ago. His previous episodes were treated with plasma exchange (PEX) and steroids, but he received 4 weekly doses of rituximab (375 mg/m2) after the most recent episode. He was rapidly started on PEX and corticosteroids for a clinical diagnosis of iTTP that was confirmed with severely deficient ADAMTS13 activity (<10%). He achieved a clinical response and was discharged from the hospital at day 11 of hospitalization. During his first outpatient visit, he had complaints of mild fatigue and was found to have thrombocytopenia with a platelet count of 65 × 109/L and a lactate dehydrogenase level (LDH) that had increased to more than 2 times the upper limit of normal. A review of his peripheral blood smear showed an increased number of schistocytes, consistent with an iTTP exacerbation.
Definition of exacerbation
The revised definition of clinical exacerbation, as proposed by an international working group, is recurrent thrombocytopenia (<150 × 109/L), with or without clinical evidence of new or progressive ischemic organ injury within 30 days of cessation of therapeutic plasma exchange or anti–von Willebrand factor (VWF) therapy, in the absence of an alternative cause of thrombocytopenia.3 This diagnosis requires an initial clinical response with sustained platelet count ≥150 × 109/L, normal LDH (<1.5 times the upper limit of normal), and no clinical evidence of new or progressive organ ischemia (Table 1). Thrombocytopenia in the first month after stopping PEX may be caused by other clinical conditions, including infection (catheter associated or not), consumptive coagulopathy, including disseminated intravascular coagulation, drug-induced thrombocytopenia, or even heparin-induced thrombocytopenia, but suspicion for an exacerbation of iTTP should be high.
This patient’s clinical picture is consistent with an exacerbation of iTTP. Exacerbations are clinically significant events that require reinitiation of PEX and intensification of immunosuppression, exposing patients to the potential morbidity and even mortality related to the recurrent iTTP and the additional treatment necessary to again achieve a clinical response.
Mechanism of exacerbations
Investigators have sought to identify risk factors for exacerbations of iTTP, and the literature suggests that demographics and ADAMTS13 activity may play a role. A study conducted by Cataland et al showed that pretreatment ADAMTS13 activity was significantly lower in patients who had an exacerbation, but after accounting for race as a covariate of ADAMTS13 activity, the difference was no longer significant, as Black patients independently had an increased risk of iTTP exacerbation (P = .046).4 Sui and colleagues also demonstrated that, whereas pretreatment ADAMTS13 activity was not predictive of exacerbation, persistently low ADAMTS13 levels (<10 U/dL; hazard ratio [HR], 4.4; P = .005) or higher anti-ADAMTS-13 IgG (HR, 3.1; P = .016, measured 3 to 7 days after initiation of PEX and immunosuppression, was predictive of exacerbation. Wu et al reported similar findings where patients with more rapid recovery of ADAMTS13 activity during PEX therapy did not experience exacerbations.5 Similarly, a low ADAMTS13 level (<10 U/dL; HR, 4.8; P = .002), low ADAMTS-13 antigen (<25th percentile; HR, 3.3; P = .01), or high anti-ADAMTS13 IgG (>75th percentile; HR, 2.6; P = .047) at the time of clinical response was predictive of exacerbation.6 Alterations in the titer and potency of the anti-ADAMTS13 antibodies have also been reported, despite daily PEX therapy and concurrent corticosteroid therapy, and could lead to both refractoriness to treatment and an increased risk of exacerbation of iTTP.7 In the first caplacizumab study, 10 of 12 subjects also had severely deficient ADAMTS13 activity (<10%) at the time they experienced an exacerbation.8
Treatment to prevent exacerbations
The only treatment clearly shown to reduce exacerbation rates is caplacizumab. Intensification of immunosuppressive therapy may be expected to reduce exacerbations, but studies of cyclosporine (CSA), steroids, and rituximab as adjuncts to PEX failed to significantly reduce exacerbation rates.9,10 Caplacizumab does not alter ADAMTS13 activity, inhibition, or function, but rather blocks the formation of microthrombi by binding to the A1 domain of VWF.8,11 In the initial caplacizumab study, when the drug was stopped after 30 days without consideration of the ADAMTS13 level, 7 of 8 patients developed recurrent iTTP in the first 10 days after the last dose, suggesting that caplacizumab protects patients from recurrent iTTP. Although the data clearly support the efficacy of caplacizumab in preventing exacerbations, questions have been raised regarding the cost effectiveness of the drug.12
Postdischarge follow-up and exacerbation risk
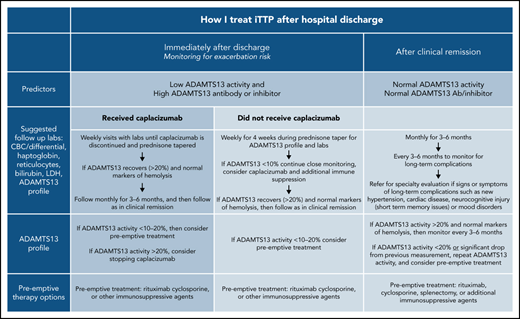
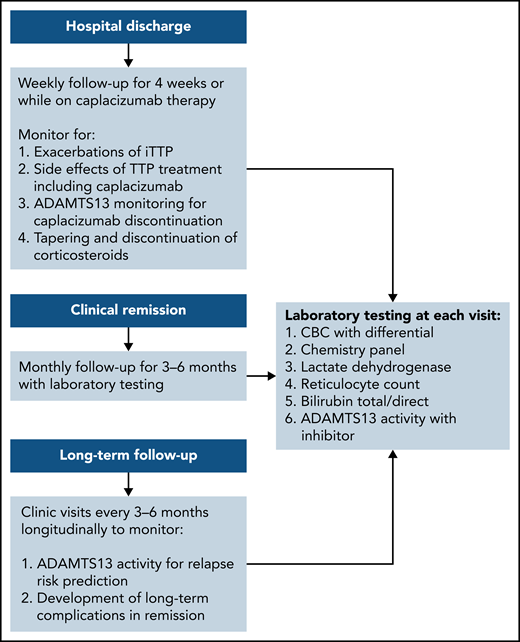
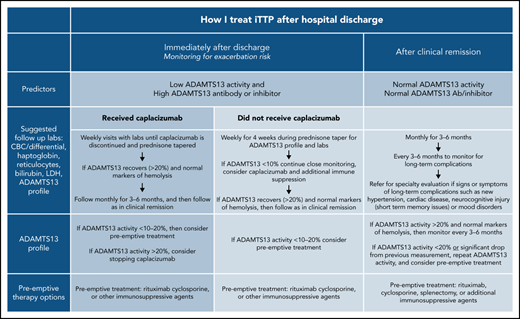
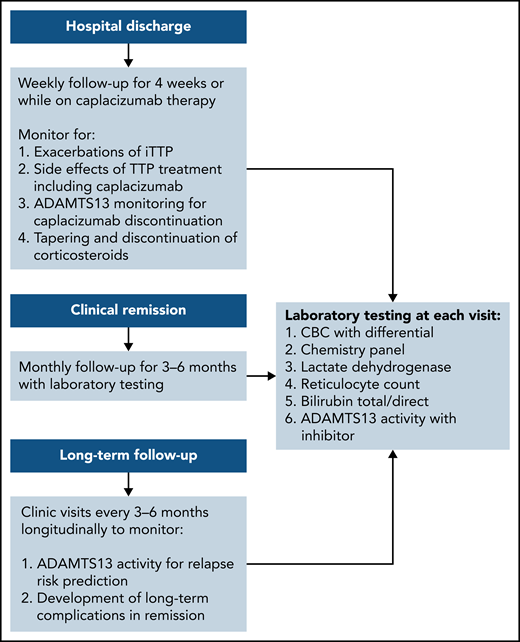
The exacerbation rate after treatment with PEX and immune suppression has been studied in 2 consecutive randomized, controlled studies and is estimated to be between 28% and 38%.8,11 The consensus protocol of the United States Thrombotic Microangiopathy (USTMA) Consortium institutions is to evaluate patients weekly for the first 4 weeks after hospital discharge to monitor for exacerbations that are most common in the first 2 weeks.13 We monitor recovery of organ injury from the acute iTTP episode clinically and with the following laboratory testing: complete blood count with differential, chemistry panel, LDH level, and reticulocyte count. Prednisone is rapidly tapered over the first 4 weeks after discharge. We use rituximab in patients with newly diagnosed and relapsed iTTP to prevent or delay relapses consistent with the recently published ISTH TTP Guidelines.14 Rituximab may be initiated during hospitalization concurrently with PEX, or alternatively, the first weekly dose can be given soon after hospital discharge. The laboratory studies obtained at these initial follow-up visits and future visits are shown in Figure 1. Patients treated with caplacizumab may have weekly visits extended beyond 4 weeks to monitor the ADAMTS13 level as they continue to receive therapy. Once patients have safely achieved clinical remission of iTTP, we monitor them monthly for 3 to 6 months followed by longitudinal visits every 3 to 6 months. The unique monitoring of caplacizumab-treated patients is discussed later.
Post–hospital discharge follow-up schedule. To monitor for exacerbations of iTTP and discontinuation of caplacizumab and long-term follow-up to predict the risk for relapse and the development of long-term complications of iTTP.
Post–hospital discharge follow-up schedule. To monitor for exacerbations of iTTP and discontinuation of caplacizumab and long-term follow-up to predict the risk for relapse and the development of long-term complications of iTTP.
Case 2
A 46-year-old Black woman was brought to the hospital by her husband with symptoms of a headache and worsening confusion over the past 2 days. She was found to have a platelet count of 6 × 109/L and a peripheral smear that showed marked fragmentation of red cells and confirmation of severe thrombocytopenia. Clinical suspicion for a diagnosis of iTTP was high; therefore, PEX was rapidly initiated in addition to prednisone at a dose of 1 mg/kg daily orally with a plan to start a course of 4 weekly rituximab treatments before discharge from the hospital. Given the high clinical suspicion for a diagnosis of iTTP and the absence of any bleeding symptoms, therapy with caplacizumab was initiated concurrently with PEX, beginning on the second day. ADAMTS13 activity was reported to be <10% on the third day of hospitalization, confirming the diagnosis of iTTP. After 6 daily PEX procedures, the platelet count and LDH level normalized, and plans for postdischarge follow-up were made.
Exacerbation risk, caplacizumab, and postdischarge follow-up
The addition of caplacizumab to PEX and steroids significantly reduces the exacerbation rate, but without alteration of the underlying immune-mediated clearance or blockage of ADAMTS13 protease function. Increasingly, rituximab is added to the initial therapy of iTTP to achieve a more durable suppression of ADAMTS13 autoantibodies and safer discontinuation of caplacizumab. In the TITAN study, discontinuation of caplacizumab at the end of the planned 30-day treatment led to disease recurrence in 8 subjects, with 7 recurrences within a week of cessation.8 Each of these 7 subjects had ADAMTS13 activity that was persistently <10%. This outcome to the treatment modification in the HERCULES study that allowed for up to 4 weeks of additional caplacizumab, whereas immune suppression was either added or intensified to allow time for recovery of ADAMTS13 activity and for safer discontinuation of caplacizumab.11
In addition to prompting changes to the definition of exacerbation of iTTP,3 these data have led to changes in the postdischarge follow-up of patients treated with caplacizumab. Although non–caplacizumab-treated patients have been seen weekly for 4 weeks after hospital discharge to monitor for exacerbations, we still follow up caplacizumab-treated patients weekly until they have discontinued caplacizumab after recovery of ADAMTS13 activity. Expert consensus has been consistent in using at least 2 weekly ADAMTS13 activity measurements (obtained at least 1 week from the last PEX) of >20% as a reasonable criterion to safely stop caplacizumab. However, a retrospective analysis of 60 patients treated with caplacizumab in Germany showed that, in 31 patients, stopping caplacizumab before 30 days and before ADAMTS13 activity was ≥10% did not result in an exacerbation of iTTP.15 Determinations of length of therapy with caplacizumab must also take into account bleeding complications.
The patients in case 2 completed 4 weekly doses of rituximab at a dose of 375 mg/m2 and at this writing is 7 weeks out from her hospital discharge and has completed 8 weeks of caplacizumab therapy. The most recent measurement of ADAMTS13 activity showed persistently deficient activity of <10% despite normal complete blood count and LDH and serum creatinine levels.
Caplacizumab discontinuation without recovery of ADAMTS13 activity
Although in most patients with acute iTTP, treatment with PEX, corticosteroids, and rituximab corrects the severely deficient ADAMTS13 level, there are patients in whom ADAMTS13 will not recover after a 4-week course of rituximab.16,17 Although severely deficient ADAMTS13 increases the risk of exacerbation or relapse of iTTP, recovery is not necessary to achieve a sustained clinical remission of iTTP. In the case of patients not treated with rituximab, it would be reasonable to consider therapy with rituximab. This therapy may be especially effective in patients for whom this event was a relapse of previously diagnosed iTTP.14
In the patient described in case 2 with newly diagnosed iTTP in whom ADAMTS13 protease function did not recover after treatment with rituximab, the decision to stop caplacizumab may not be so straightforward. The best available data regarding the timing of the recovery of ADAMTS13 activity after acute iTTP treatment were reported by Scully et al where the first dose of rituximab was given before the third day of hospitalization.9 When measured before the second rituximab infusion 1 week later, median ADAMTS13 activity was 15%. After 8 weeks, the median was >30%, increasing to a median of 45% after 3 months. These data suggest using a time frame of 8 to 12 weeks from the start of rituximab to assess its efficacy at improving ADAMTS13 activity. Interestingly, Barba et al reported data on a subset of patients with iTTP in whom ADAMTS13 activity failed to improve after an initial 4-week course of preemptive rituximab (375 mg/m2). In this cohort, patients subsequently received intensive rituximab therapy at a dose of 375 mg/m2 every 2 to 3 months for a median of 2 years (range, 1-6). Ten of the 13 patients improved their ADAMTS13 activity a median of 56 days (range, 10 days to 2 years) after starting the intensive regimen.18 In patients in whom their ADAMTS13 activity failed to improve after treatment with rituximab, additional immunosuppressive therapies could be considered (see later description of preemptive therapy). However, additional immunosuppressive therapy after rituximab, such as CSA, bortezomib, mycophenolate mofetil, and alemtuzumab, may require long-term treatment or have potentially unfavorable side effects.19
There are unfortunately no published data to clearly guide physicians. In these situations, our approach has been to consider additional immunosuppressive therapy in those with a history of relapses, where a relapsing clinical phenotype has been demonstrated and where the risk-benefit ratio may favor the addition of another immunosuppressive therapy. A discussion about the risks and benefits of additional immunosuppressive therapy should be undertaken with patients in these situations. In the event that caplacizumab is stopped without recovery of ADAMTS13 activity, weekly or even biweekly labs should be considered for at least the first 2 to 4 weeks to monitor for exacerbations of iTTP. Given that this was the initial iTTP episode, caplacizumab was stopped after 8 weeks of therapy, and the patient was monitored closely. Despite persistently deficient ADAMTS13 activity in remission, she maintained a continuous clinical remission and continued to be observed every 3 months.
The 36-year-old white man described in case 1 with a history of chronic, relapsing iTTP was able to achieve a clinical remission and a complete ADAMTS13 remission after 4 weekly infusions of rituximab. He presented to the outpatient clinic 3 months after completing rituximab to discuss his risk of relapse and any measures that could be taken to reduce this risk. He also had questions regarding persistent headaches and short-term memory problems that he had recently noted.
Risk of relapse
Relapse is a major concern for patients with iTTP after recovery from an acute iTTP episode.20 Approximately 50% or more patients not treated with rituximab have at least 1 relapse within 5 years of initial diagnosis. Although prediction of relapse is inexact, previous relapse of iTTP, severely deficient ADAMTS13 activity in remission, and anti-ADAMTS13 autoantibodies during remission have been associated with an increased risk of iTTP relapse.16,21-24 In addition, young age at presentation and previous episode of iTTP have been shown to be predictive of relapse.21,22,25 Race may also be an important factor. A large retrospective study of the USTMA Consortium database showed that Black race was the strongest predictor of relapse.26 In relapsed iTTP, relapse-free survival was significantly better in White patients after rituximab, but treatment with rituximab did not improve relapse-free survival in Black patients in this study. These data suggest that race may differentially impact responses to preemptive immunosuppressive therapy.
Prediction of relapse is clearly more complicated than ADAMTS13 activity alone. Circulating ultralarge VWF multimers correlated with severely deficient ADAMTS13 activity, but the relationship was imprecise.27 Masias et al developed a multivariable model to predict relapses of iTTP in the following 3 months after remission blood testing. Additional factors including age, LDH, C3a, ADAMTS13 antigen, and the presence of ultralarge multimers were able to improve the prediction of relapse over ADAMTS13 activity alone, but the optimal clinical application of this model requires further study.28
Monitoring ADAMTS13 activity in remission
After achieving clinical remission of iTTP, the follow-up and monitoring strategy is then focused on the prevention of relapse. Although the optimal monitoring schedule remains to be defined, our practice is to monitor the complete blood count, chemistry, and markers of hemolysis (LDH and haptoglobin) every 3 months for longitudinal follow-up.29 It is also our practice to measure ADAMTS13 activity every 3 months to help assess the risk of clinical and ADAMTS13 relapse (Figure 1).3 This schedule was not developed from prospective study, but rather was derived in part from a study by Jin et al that reported the risk of relapse in the 3 months after measurement of ADAMTS13 activity.21 Experts have also agreed that monitoring ADAMTS13 every 3 months allows for sufficient time to intervene with preemptive therapy, while not being overly onerous to patients. After several years of continuous clinical and ADAMTS13 remission, it may be reasonable to alter the schedule to every 6 months for patients with difficulties keeping this rigorous follow-up schedule, but maintaining a consistent follow-up plan is very important. In addition, patients must be monitored for the development of long-term complications of iTTP that have been associated with premature death in at least 2 studies.1,2
ADAMTS13 activity threshold for preemptive treatment
Although preemptive therapy with rituximab has been shown to prevent or delay relapses of iTTP,16,17 there are no randomized, prospective data to accurately define a specific threshold for ADAMTS13 activity when preemptive immune suppression should be initiated. Moreover, the development of severely deficient ADAMTS13 activity does not uniformly lead to relapse and may fluctuate in remission.21,23,30 Given the current knowledge of the efficacy and favorable side effect profile of rituximab, the threshold we typically use to initiate preemptive treatment has increased from ADAMTS13 activity of ≤10% to a threshold of 20%. This level is based on expert opinion regarding the increased risk of relapse with decreasing ADAMTS13 activity and the need to allow time for response to immunosuppressive therapy rather than prospective data. Repeat measurement of ADAMTS13 activity in the following 2 to 4 weeks is recommended for those with activity >20% or those with a decrease from the previous measurement, to establish a trend. In the absence of an absolute threshold to initiate preemptive therapy, the risk and complications of an iTTP relapse must be balanced against the risk of any preemptive therapy. As stated previously, younger patients and patients with prior relapses are likely to be at greater risk of relapse, which should also factor into the decision making.
Preemptive treatment to prevent relapse
A detailed discussion of every preemptive agent reported is beyond the scope of this manuscript, but preemptive therapies that are commonly used and supported by data will be discussed. The most common preemptive treatment used is rituximab, but there are also data regarding the use of CSA and splenectomy during remission to prevent relapse. There are reports of other immunosuppressive therapies, including alemtuzumab, bortezomib, cyclophosphamide, or mycophenolate, that have been used to prevent relapse and could be considered, depending on the individual circumstances of the patient.
Rituximab
The efficacy and safety of rituximab has been established in several observational studies of patients with acute, refractory, or relapsed iTTP.31-33 Reduction of relapses with the use of preemptive rituximab has been demonstrated by several groups as well.31-33 The French Thrombotic Microangiopathy Reference Center studied 92 patients with iTTP in remission with undetectable ADAMTS13 activity (<10%). During a follow-up period of 35.8 months, rituximab significantly decreased the median number of iTTP relapses (0 episodes per year vs 0.33 episodes per year compared with historical controls).16 The optimal dose and schedule of rituximab has not been determined in iTTP, but the most common dose currently used is 375 mg/m2 per week for 4 weeks. The need for additional courses of rituximab beyond the initial treatment should be guided by regular, serial measurements of ADAMTS13 activity. Preemptive rituximab on a fixed, regular schedule (every 3 months) without the guidance of serial monitoring of ADAMTS13 activity is discouraged, as it could lead to unnecessary rituximab therapy.14
CSA
Several reports have described the use of cyclosporine (CSA) for iTTP including as a preemptive treatment to prevent relapse in patients with severely deficient ADAMTS13 activity and a history of multiple recurrences (>2).10,34-38 The dose administered for prophylaxis was 2 to 3 mg/kg orally twice daily in divided doses for a total of 6 months. Trough CSA levels were used in this study to monitor for toxicity, but levels were not correlated with response to therapy. At this lower dose, the CSA was well tolerated, but patients must be counseled regarding side effects that include gingival hyperplasia, renal insufficiency, and increased hair growth. Data from 19 patients indicated that that CSA is effective in suppressing ADAMTS13 inhibitors and in improving ADAMTS13 activity over the 6-month study, but 7 of 19 patients relapsed after discontinuation of CSA, suggesting that the benefit does not persist beyond the CSA treatment period.39,40 If used preemptively, CSA is likely to be administered over a prolonged period. As with rituximab, the level of ADAMTS13 activity should be used to judge the efficacy of CSA with drug levels used only to monitor for toxicity.
Splenectomy
A systematic review of 18 studies of splenectomy in iTTP demonstrated that the relapse rate was reduced to 17% in a cohort of patients with a chronic, relapsing phenotype. Despite the limitations (no control groups and possible publication bias), the response rate after splenectomy at 3 years ranged between 70% and 80%.41 In another series of patients who underwent splenectomy preemptively to prevent relapse, the relapse rate fell from 0.74 relapses per patient-year to 0.1 relapse per patient-year.42 Splenectomy, however, should not be considered in patients with acute, refractory iTTP, given the greater risk for complications and death.42 We believe that splenectomy could be considered a preemptive treatment in remission to prevent relapse of iTTP after considering other preemptive options and a thorough discussion of the risks and benefits with the patient. ADAMTS13 activity after splenectomy should be monitored as a measure of efficacy.
Long-term complications
It has been increasingly clear that a new diagnosis of iTTP is the beginning of a long-term follow-up for a disease that is associated with several long-term complications (Table 2). At least 2 studies have demonstrated a shorter life expectancy in survivors of an acute iTTP episode, with cardiovascular complications playing a significant role.1,43 Published work by Upreti et al demonstrated a fivefold increased risk for stroke in patients with iTTP (unrelated to an acute iTTP episode) in remission during long-term follow-up, with all of the patients who developed strokes having had lower-than-normal (<70%) ADAMTS13 activity.44 These data support the hypothesis that preemptive immunosuppressive therapy may help to prevent stokes in patients with iTTP in remission. Given these data, we have placed increased emphasis on addressing modifiable risk factors for cardiovascular disease including blood pressure, smoking cessation, and treatment of hypercholesterolemia, as indicated, to attempt to decrease the risk for cardiovascular disease. We also commonly recommend referral to a cardiologist for baseline evaluation and assessment of cardiovascular risk after a diagnosis of iTTP and for consideration of the use of antiplatelet therapy and other risk reduction strategies. Although data on the efficacy of these measures in patients with iTTP are lacking, antiplatelet therapy (aspirin, clopidogrel) seems reasonable in patients with iTTP at risk for cardiovascular disease as would be considered in similar nonpatients with iTTP.
Neurocognitive deficits that include short-term memory problems, mood disorders, headaches, and posttraumatic stress disorder are clearly recognized to be more common in patients with iTTP in remission.1,45-50 In a study of select patients in iTTP remission from The Ohio State University and the University College London Hospitals (n = 27), 63% of the patients studied demonstrated neurocognitive impairment, particularly in the areas of visual learning and memory.51 Deford et al also reported that nearly 20% of the iTTP survivors in their study met criteria for a diagnosis of major depression, which was significantly greater than the expected population rate of 6%.1 Another study from Chaturvedi et al, who used an online survey tool, found that 80% of patients with iTTP in remission had at least mild symptoms of depression.50 They also reported that 35% of patients with iTTP had a positive screening for posttraumatic stress disorder. It is presently unclear how to best screen for these complications in patients with iTTP to allow for interventions to mitigate the morbidity and mortality associated with them. We recommend referral to psychiatry or psychology for detailed neurocognitive testing during follow-up when there are possible signs or symptoms of mood or cognitive issues after a diagnosis of iTTP.
Pregnancy and relapse
The question of pregnancy and the risk for relapse in patients with a prior diagnosis of iTTP is understandably an important issue for patients. Severely deficient ADAMTS13 activity in pregnancy is associated with an increased risk of relapse during pregnancy.29 As a part of preconception counseling in a patient with a history of iTTP, a discussion of this risk of relapse as well as the potential need for prophylactic immunosuppressive therapy is necessary. Regular monitoring of ADAMTS13 activity is recommended every 2 to 3 months throughout the pregnancy, using the trend in the ADAMTS13 activity to assess the need for preemptive therapy to prevent relapse of iTTP.
Authorship
Contribution: F.A., A.A., and S.R.C. designed and wrote the manuscript.
Conflict-of-interest-disclosure: S.R.C. has received research funding and consulting fees from Sanofi. F.A. and A.A. declare no competing financial interests.
Correspondence: Spero R. Cataland, Department of Hematology, The Ohio State University, 1150F Lincoln Tower, 1800 Cannon Dr, Columbus, OH 43210; e-mail: spero.cataland@osumc.edu.