Key Points
In the era of the Omicron variant of COVID-19, lower fatality rates in CLL are seen along with milder disease in the background population.
Patients with CLL who have hospital contact and test positive for SARS-CoV-2 should still be considered for preemptive therapy.
Abstract
Previous studies have shown that patients with chronic lymphocytic leukemia (CLL) and coronavirus disease 2019 (COVID-19) have high mortality rates. Infection with the Omicron variant has been described as a milder disease course in the general population. However, the outcome for immunocompromised patients has not previously been reported. In a cohort of patients with CLL tested for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) at hospital test sites in the time periods before and after dominance of the Omicron variant, rates of hospitalizations and intensive care unit admissions declined significantly, whereas 30-day mortality remained as high as 23% in the period with dominance of the Omicron sublineage BA.2 variant. However, for a larger population-based cohort of patients with CLL (including the hospital cohort), 30-day mortality was 2%. Thus, patients with CLL with close hospital contacts and, in particular, those >70 years of age with 1 or more comorbidities should be considered for closer monitoring and preemptive antiviral therapy upon a positive SARS-CoV-2 test.
Introduction
The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) B.1.1.529 (Omicron) variant of coronavirus disease 2019 (COVID-19) is reported to give milder disease in the general population; outcomes for immunocompromised patients have not been reported. Here, hospital- and population-based data on outcome for patients with chronic lymphocytic leukemia (CLL) upon infection with the Omicron variant of SARS-CoV-2 warrant close monitoring and preemptive therapy upon a positive SARS-CoV-2 test for patients with CLL and frequent hospital contacts; other patients with CLL can expect a mild course of COVID-19.
Patients with CLL have increased morbidity and mortality following infection with SARS-CoV-2, leading to COVID-19.1,2 The immune dysfunction inherent to CLL itself and CLL treatment, whether targeted or chemoimmunotherapy based, is considered the likely cause of increased susceptibility to severe COVID-19.3 During the first and second pandemic waves, most CLL patients with COVID-19 developed severe disease, and the 30-day mortality was 31% to 50% for those admitted, although one study indicates improved survival for patients with CLL upon COVID-19 later in the pandemic.1,2,4 Further, patients with CLL demonstrated impaired vaccination response in terms of ability to produce neutralizing anti–SARS-CoV-2 antibodies, even though the T-cell response was also impaired for part of the populations.5-8 Data on outcome upon infection with SARS-CoV-2 Omicron variant is warranted for immunocompromised patients in general and for patients with CLL in particular.9 The first Danish Omicron case was detected on 25 November 2021. The variant became dominant in Denmark by 17 December 2021, enabling high levels of breakthrough infections among vaccinated individuals.10
In Denmark, all patients diagnosed with a hematological malignancy were offered third and fourth booster vaccination against SARS-CoV-2 in August 2021 and January 2022, respectively. At the same time, a single dose of anti–SARS-CoV-2 monoclonal antibody (mAb) was recommended for immunocompromised patients testing positive for SARS-CoV-2 with sotrovimab being the most widely used mAb in Denmark, while the standard of care for immunocompromised patients admitted with moderate to severe COVID-19 was dexamethasone, low molecular weight heparin, and remdesivir.11,12 Remdesivir was widely used for hematological patients regardless of disease severity since approval mid-2020.13,14 Sotrovimab retained its neutralizing activities against the Omicron BA.1 sublineage, but recently, in vitro studies have shown reduced activity against the BA.2 sublineage.15,16
Methods
Insights into potential variation in clinical outcome for immunocompromised patients upon infection with the Omicron variant is limited. Here, we investigated the rate of hospitalization, admission to intensive care unit (ICU), and mortality following infection with SARS-CoV-2 among patients with CLL in a Danish cohort with SARS-CoV-2 polymerase chain reaction (PCR) test from electronic health records (EHR) between March 2020 and January 2022 (EHR cohort). Additionally, we analyzed a cohort of patients registered with a diagnosis of CLL in the Danish CLL registry17 for whom a positive SARS-CoV-2 PCR test was identified through the PERSIMUNE treatment database with microbiology data retrieved as previously described (population cohort).18 As data on variants were missing for most patients, we grouped patients into 4 time periods based on the first positive SARS-CoV-2 PCR: period 1: March 2020 to December 2020; period 2: January 2021 to 25 November 2021 (first Omicron case in Denmark); period 3: 26 November 2021 to 31 December 2021; and period 4: 1 January 2022 to 28 January (Omicron variant dominating from 17 December 2021 and sublineage BA.2 dominating from 1 January 2022). Data were retrieved from EHR covering a background population of ∼2.8 million individuals.19 We included all patients with a CLL diagnosis (ICD10 code DC91.1) and a positive PCR for SARS-CoV-2 within the EHR (EHR cohort). The population cohort initiates in September 2020, the time of introducing widespread testing outside the EHR. Patients with multiple positive PCR tests >12 weeks apart were considered as having reinfection. Baseline characteristics were stratified by time-period of first positive SARS-CoV-2 PCR test (Table 1). Primary outcomes were time to hospital admission, time to ICU admission, and 30-day mortality. We followed patients from date of first positive PCR until event, death or date of last follow-up (22 February 2022 and 15 March 2022 for the EHR and population cohort, respectively). The study was approved by the ethics committee and data protection agency.
Patient characteristics, stratified on cohort (EHR or population) and period of COVID-19+ test
| . | Period 1 . | Period 2 . | Period 3 . | Period 4 . | . | . | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Covariate . | EHR (n = 59) . | Population (n = 24) . | EHR (n = 40) . | Population (n = 66) . | EHR (n = 32) . | Population (n = 73) . | EHR (n = 22) . | Population (n = 477) . | Total (n = 793) . | P value . | |
| Age at PCR | Median [IQR] | 71 [64.5, 80.5] | 70.5 [54.2, 74.5] | 77 [68.2, 82.0] | 70 [62, 78] | 74.5 [69.8, 83.0] | 70 [63, 77] | 76 [72.0, 80.5] | 72 [64, 77] | 72 [64, 78] | .0052 |
| Sex | Female | 27 (45.8) | 7 (29.2) | 17 (42.5) | 24 (36.4) | 12 (37.5) | 29 (39.7) | 9 (40.9) | 181 (37.9) | 306 (38.6) | |
| Male | 32 (54.2) | 17 (70.8) | 23 (57.5) | 42 (63.6) | 20 (62.5) | 44 (60.3) | 13 (59.1) | 296 (62.1) | 487 (61.4) | .91 | |
| CLL diagnosis | Median [IQR] | 2017 [2012, 2019] | 2015 [2012, 2016] | 2015 [2010, 2018] | 2015 [2013, 2016] | 2016 [2014, 2018] | 2015 [2013, 2017] | 2017 [2014, 2018] | 2015 [2012, 2017] | 2015 [2012, 2017] | .024 |
| Missing | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 3 | ||
| Binet stage | A | 34 (87.2) | 20 (83.3) | 22 (75.9) | 57 (86.4) | 21 (95.5) | 69 (94.5) | 14 (73.7) | 402 (84.3) | 639 (85.3) | |
| B | 3 (7.7) | 2 (8.3) | 6 (20.7) | 7 (10.6) | 1 (4.5) | 4 (5.5) | 3 (15.8) | 65 (13.6) | 91 (12.1) | ||
| C | 2 (5.1) | 2 (8.3) | 1 (3.4) | 2 (3.0) | 0 (0.0) | 0 (0.0) | 2 (10.5) | 10 (2.1) | 19 (2.5) | .097 | |
| Missing | 20 | 0 | 11 | 0 | 10 | 0 | 3 | 0 | 44 | ||
| IGHV status | Unmutated | 8 (28.6) | 6 (33.3) | 9 (39.1) | 18 (33.3) | 6 (40.0) | 19 (33.9) | 3 (20.0) | 105 (27.0) | 174 (29.1) | |
| Mutated | 20 (71.4) | 12 (66.7) | 14 (60.9) | 36 (66.7) | 9 (60.0) | 37 (66.1) | 12 (80.0) | 284 (73.0) | 424 (70.9) | .70 | |
| Missing | 31 | 6 | 17 | 12 | 17 | 17 | 7 | 88 | 195 | ||
| FISH status | Del13q | 18 (64.3) | 11 (64.7) | 10 (43.5) | 28 (54.9) | 9 (50.0) | 34 (61.8) | 7 (46.7) | 221 (61.6) | 338 (59.7) | |
| Normal | 3 (10.7) | 1 (5.9) | 4 (17.4) | 9 (17.6) | 0 (0.0) | 9 (16.4) | 1 (6.7) | 44 (12.3) | 71 (12.5) | ||
| Tri12 | 4 (14.3) | 3 (17.6) | 3 (13.0) | 6 (11.8) | 5 (27.8) | 9 (16.4) | 3 (20.0) | 53 (14.8) | 86 (15.2) | ||
| Del11q | 1 (3.6) | 1 (5.9) | 3 (13.0) | 5 (9.8) | 2 (11.1) | 3 (5.5) | 3 (20.0) | 24 (6.7) | 42 (7.4) | ||
| Del17p | 2 (7.1) | 1 (5.9) | 3 (13.0) | 3 (5.9) | 2 (11.1) | 0 (0.0) | 1 (6.7) | 17 (4.7) | 29 (5.1) | .68 | |
| Missing | 31 | 7 | 17 | 15 | 14 | 18 | 7 | 118 | 227 | ||
| Admission | Yes | 41 (69.5) | NA | 33 (82.5) | NA | 19 (59.4) | NA | 12 (54.5) | NA | 105 (68.6) | |
| No | 18 (30.5) | NA | 7 (17.5) | NA | 13 (40.6) | NA | 10 (45.5) | NA | 48 (31.4) | .075 | |
| Missing | 0 | 24 | 0 | 66 | 0 | 73 | 0 | 477 | 640 | ||
| ICU | Yes | 7 (11.9) | NA | 5 (12.5) | NA | 1 (3.1) | NA | 0 (0.0) | NA | 13 (8.5) | |
| No | 52 (88.1) | NA | 35 (87.5) | NA | 31 (96.9) | NA | 22 (100.0) | NA | 140 (91.5) | .26 | |
| Missing | 0 | 24 | 0 | 66 | 0 | 73 | 0 | 477 | 640 | ||
| Died | Yes | 10 (16.9) | 0 (0.0) | 7 (17.5) | 4 (6.1) | 3 (9.4) | 4 (5.5) | 5 (22.7) | 4 (0.8) | 37 (4.7) | |
| No | 49 (83.1) | 24 (100.0) | 33 (82.5) | 62 (93.9) | 29 (90.6) | 69 (94.5) | 17 (77.3) | 473 (99.2) | 756 (95.3) | <.0001 | |
| Dexa-methasone | Yes | 11 (18.6) | NA | 16 (40.0) | NA | 9 (28.1) | NA | 4 (18.2) | NA | 40 (26.1) | |
| No | 48 (81.4) | NA | 24 (60.0) | NA | 23 (71.9) | NA | 18 (81.8) | NA | 113 (73.9) | .090 | |
| Missing | 0 | 24 | 0 | 66 | 0 | 73 | 0 | 477 | 640 | ||
| Remdesivir | Yes | 12 (20.3) | NA | 18 (45.0) | NA | 8 (25.0) | NA | 3 (13.6) | NA | 41 (26.8) | |
| No | 47 (79.7) | NA | 22 (55.0) | NA | 24 (75.0) | NA | 19 (86.4) | NA | 112 (73.2) | .019 | |
| Missing | 0 | 24 | 0 | 66 | 0 | 73 | 0 | 477 | 640 | ||
| mAb | Yes | 0 (0.0) | NA | 10 (25.0) | NA | 12 (37.5) | NA | 8 (36.4) | NA | 30 (19.6) | |
| No | 59 (100.0) | NA | 30 (75.0) | NA | 20 (62.5) | NA | 14 (63.6) | NA | 123 (80.4) | <.0001 | |
| Missing | 0 | 24 | 0 | 66 | 0 | 73 | 0 | 477 | 640 | ||
| . | Period 1 . | Period 2 . | Period 3 . | Period 4 . | . | . | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Covariate . | EHR (n = 59) . | Population (n = 24) . | EHR (n = 40) . | Population (n = 66) . | EHR (n = 32) . | Population (n = 73) . | EHR (n = 22) . | Population (n = 477) . | Total (n = 793) . | P value . | |
| Age at PCR | Median [IQR] | 71 [64.5, 80.5] | 70.5 [54.2, 74.5] | 77 [68.2, 82.0] | 70 [62, 78] | 74.5 [69.8, 83.0] | 70 [63, 77] | 76 [72.0, 80.5] | 72 [64, 77] | 72 [64, 78] | .0052 |
| Sex | Female | 27 (45.8) | 7 (29.2) | 17 (42.5) | 24 (36.4) | 12 (37.5) | 29 (39.7) | 9 (40.9) | 181 (37.9) | 306 (38.6) | |
| Male | 32 (54.2) | 17 (70.8) | 23 (57.5) | 42 (63.6) | 20 (62.5) | 44 (60.3) | 13 (59.1) | 296 (62.1) | 487 (61.4) | .91 | |
| CLL diagnosis | Median [IQR] | 2017 [2012, 2019] | 2015 [2012, 2016] | 2015 [2010, 2018] | 2015 [2013, 2016] | 2016 [2014, 2018] | 2015 [2013, 2017] | 2017 [2014, 2018] | 2015 [2012, 2017] | 2015 [2012, 2017] | .024 |
| Missing | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 3 | ||
| Binet stage | A | 34 (87.2) | 20 (83.3) | 22 (75.9) | 57 (86.4) | 21 (95.5) | 69 (94.5) | 14 (73.7) | 402 (84.3) | 639 (85.3) | |
| B | 3 (7.7) | 2 (8.3) | 6 (20.7) | 7 (10.6) | 1 (4.5) | 4 (5.5) | 3 (15.8) | 65 (13.6) | 91 (12.1) | ||
| C | 2 (5.1) | 2 (8.3) | 1 (3.4) | 2 (3.0) | 0 (0.0) | 0 (0.0) | 2 (10.5) | 10 (2.1) | 19 (2.5) | .097 | |
| Missing | 20 | 0 | 11 | 0 | 10 | 0 | 3 | 0 | 44 | ||
| IGHV status | Unmutated | 8 (28.6) | 6 (33.3) | 9 (39.1) | 18 (33.3) | 6 (40.0) | 19 (33.9) | 3 (20.0) | 105 (27.0) | 174 (29.1) | |
| Mutated | 20 (71.4) | 12 (66.7) | 14 (60.9) | 36 (66.7) | 9 (60.0) | 37 (66.1) | 12 (80.0) | 284 (73.0) | 424 (70.9) | .70 | |
| Missing | 31 | 6 | 17 | 12 | 17 | 17 | 7 | 88 | 195 | ||
| FISH status | Del13q | 18 (64.3) | 11 (64.7) | 10 (43.5) | 28 (54.9) | 9 (50.0) | 34 (61.8) | 7 (46.7) | 221 (61.6) | 338 (59.7) | |
| Normal | 3 (10.7) | 1 (5.9) | 4 (17.4) | 9 (17.6) | 0 (0.0) | 9 (16.4) | 1 (6.7) | 44 (12.3) | 71 (12.5) | ||
| Tri12 | 4 (14.3) | 3 (17.6) | 3 (13.0) | 6 (11.8) | 5 (27.8) | 9 (16.4) | 3 (20.0) | 53 (14.8) | 86 (15.2) | ||
| Del11q | 1 (3.6) | 1 (5.9) | 3 (13.0) | 5 (9.8) | 2 (11.1) | 3 (5.5) | 3 (20.0) | 24 (6.7) | 42 (7.4) | ||
| Del17p | 2 (7.1) | 1 (5.9) | 3 (13.0) | 3 (5.9) | 2 (11.1) | 0 (0.0) | 1 (6.7) | 17 (4.7) | 29 (5.1) | .68 | |
| Missing | 31 | 7 | 17 | 15 | 14 | 18 | 7 | 118 | 227 | ||
| Admission | Yes | 41 (69.5) | NA | 33 (82.5) | NA | 19 (59.4) | NA | 12 (54.5) | NA | 105 (68.6) | |
| No | 18 (30.5) | NA | 7 (17.5) | NA | 13 (40.6) | NA | 10 (45.5) | NA | 48 (31.4) | .075 | |
| Missing | 0 | 24 | 0 | 66 | 0 | 73 | 0 | 477 | 640 | ||
| ICU | Yes | 7 (11.9) | NA | 5 (12.5) | NA | 1 (3.1) | NA | 0 (0.0) | NA | 13 (8.5) | |
| No | 52 (88.1) | NA | 35 (87.5) | NA | 31 (96.9) | NA | 22 (100.0) | NA | 140 (91.5) | .26 | |
| Missing | 0 | 24 | 0 | 66 | 0 | 73 | 0 | 477 | 640 | ||
| Died | Yes | 10 (16.9) | 0 (0.0) | 7 (17.5) | 4 (6.1) | 3 (9.4) | 4 (5.5) | 5 (22.7) | 4 (0.8) | 37 (4.7) | |
| No | 49 (83.1) | 24 (100.0) | 33 (82.5) | 62 (93.9) | 29 (90.6) | 69 (94.5) | 17 (77.3) | 473 (99.2) | 756 (95.3) | <.0001 | |
| Dexa-methasone | Yes | 11 (18.6) | NA | 16 (40.0) | NA | 9 (28.1) | NA | 4 (18.2) | NA | 40 (26.1) | |
| No | 48 (81.4) | NA | 24 (60.0) | NA | 23 (71.9) | NA | 18 (81.8) | NA | 113 (73.9) | .090 | |
| Missing | 0 | 24 | 0 | 66 | 0 | 73 | 0 | 477 | 640 | ||
| Remdesivir | Yes | 12 (20.3) | NA | 18 (45.0) | NA | 8 (25.0) | NA | 3 (13.6) | NA | 41 (26.8) | |
| No | 47 (79.7) | NA | 22 (55.0) | NA | 24 (75.0) | NA | 19 (86.4) | NA | 112 (73.2) | .019 | |
| Missing | 0 | 24 | 0 | 66 | 0 | 73 | 0 | 477 | 640 | ||
| mAb | Yes | 0 (0.0) | NA | 10 (25.0) | NA | 12 (37.5) | NA | 8 (36.4) | NA | 30 (19.6) | |
| No | 59 (100.0) | NA | 30 (75.0) | NA | 20 (62.5) | NA | 14 (63.6) | NA | 123 (80.4) | <.0001 | |
| Missing | 0 | 24 | 0 | 66 | 0 | 73 | 0 | 477 | 640 | ||
Period 1: 12 March and 16 September 2020 for EHR and population cohorts, respectively, to 31 December 2020; period 2: January 2021 to 25 November 2021; period 3: 26 November 2021 to December 2021; and period 4: 1 January 2022 to 28 January 2022 and 7 March 2022 for EHR and population cohorts, respectively. Data on monoclonal antibodies against COVID-19 (mAb), remdesivir, and dexamethasone treatment upon COVID-19 for the different time periods were only available for the EHR cohort. Continuous variables summarized with median and interquartile range (IQR) were tested using Kruskal-Wallis tests, whereas categorical variables summarized by count (percentage) were tested using χ-square tests for differences across all 8 subgroups. P values were calculated using the log-rank test.
Results and discussion
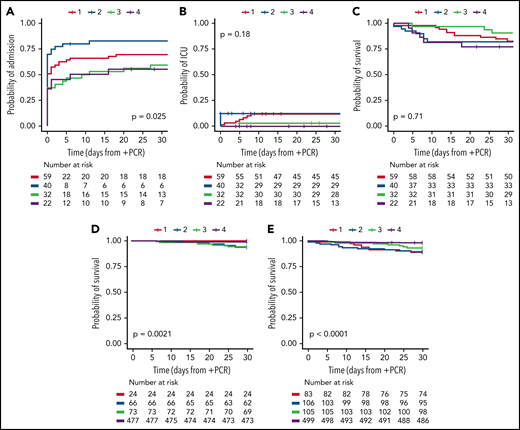
Until 28 January 2022, 151 patients with CLL had 153 COVID-19 infections confirmed with a positive PCR test for SARS-CoV-2 in the EHR system for Eastern Denmark (EHR cohort). Two reinfections were identified with positive PCR tests >1 year apart. Additionally, we identified 640 patients within the Danish CLL registry with a positive SARS-CoV-2 PCR test outside the EHR system (population cohort). No reinfections in terms of patients with positive PCR tests >12 weeks apart were identified within this cohort (patients within the EHR cohort were excluded from the population cohort). Stratified by period, 59, 40, 32, and 22 patients in the EHR cohort were first PCR positive in time periods 1 to 4, respectively. In the population cohort, 24, 66, 73, and 477 patients were first PCR positive in periods 1 to 4, respectively. There were no significant differences in baseline characteristics between the 4 periods, but patients in the EHR cohort were significantly older compared with patients in the population cohort (P = .0052), even though the patients in the EHR cohort were also diagnosed with CLL significantly more recently (Table 1; P = .024). At least 43 of 109 (39%) and 190 of 640 (30%) patients in the EHR and population cohort, respectively, had received CLL therapy prior to testing positive for SARS-CoV-2 (P=.054. For the EHR cohort, the rate of hospitalizations for patients with CLL testing positive for SARS-CoV-2 was significantly higher (>75%) during the second period compared with periods 3 (Omicron emergence) and 4 (Omicron dominance), where preemptive mAb were administered during hospital admissions for patients with CLL upon a positive SARS-CoV-2 PCR test (Figure 1A; P < .014). During period 3 and 4, mAb were administered at outpatient visits, which likely explains the lower 30-day admission rates (56% to 60% vs 83%). ICU admission rates were highest prior to emergence of Omicron (12% to 12.5% vs 0% to 3%, Figure 1B), which may reflect impact of a third and fourth booster vaccine, improved care for patients with COVID-19, and differences in severity between SARS-CoV-2 variants.11,12,20 The ICU admission rates were lower than previously reported in international cohorts of COVID-19 in CLL (26% to 37% for hospitalized patients).1,2 This could be due to the full implementation of early treatment with mAb, almost universal treatment with remdesivir for hospitalized patients without renal failure, and high vaccination rates and administration of up to 30 L/min oxygen outside the ICU in Denmark.
Admission to hospital, admission to ICU, and overall survival upon COVID-19 in CLL. Kaplan-Meier curves for (A) admission to hospital, (B) admission to ICU, (C) overall survival (OS) for the EHR cohort, (D) OS for the population cohort, and (E) OS for the combined cohort. Data are stratified for the following time periods: period 1: 12 March and 16 September 2020 for EHR and population cohorts, respectively, to December 2020; period 2: January 2021 to 25 November 2021; period 3: 26 November 2021 to December 2021; and period 4: 1 January 2022 to 28 January 2022 and 7 March 2022 for EHR and population cohorts, respectively. Patients represented within the EHR cohort (A-C) are excluded from the population cohort (D). P values were calculated using log-rank test for differences across the 4 subgroups.
Admission to hospital, admission to ICU, and overall survival upon COVID-19 in CLL. Kaplan-Meier curves for (A) admission to hospital, (B) admission to ICU, (C) overall survival (OS) for the EHR cohort, (D) OS for the population cohort, and (E) OS for the combined cohort. Data are stratified for the following time periods: period 1: 12 March and 16 September 2020 for EHR and population cohorts, respectively, to December 2020; period 2: January 2021 to 25 November 2021; period 3: 26 November 2021 to December 2021; and period 4: 1 January 2022 to 28 January 2022 and 7 March 2022 for EHR and population cohorts, respectively. Patients represented within the EHR cohort (A-C) are excluded from the population cohort (D). P values were calculated using log-rank test for differences across the 4 subgroups.
For the EHR cohort, 30-day OS was above 75% in all 4 periods (77% to 91%, Figure 1C). Despite representing a cohort with close hospital connection (EHR cohort), these survival rates are slightly better than the previously reported OS rates for COVID-19 in CLL during the first part of the pandemic (64% to 73%), although one study reported a higher OS rate of 89% for CLL patients testing positive for SARS-CoV-2 after 1 May 2020.1,2 Five out of 6 fatal cases (including deaths after 30 days) in period 3 were infected with the δ variant (missing variant information for the last case, data not shown). The 5 patients who died within 30 days of a positive SARS-CoV-2 test in period 4 were aged above 71 years, and all had comorbidities (eg, dementia, other malignant diseases, diabetes, cardiac and pulmonary comorbidities). Four of the 5 fatal cases had confirmed Omicron variant, while variant data were missing for the last case. Three of the 5 patients died of respiratory failure, and 2 patients died at home without known cause of death. Two of the fatal cases received mAb and dexamethasone and 1 of them also remdesivir; the 3 remaining fatal cases did not receive COVID-19–specific treatment. To assess whether the EHR cohort was biased toward patients with more severe COVID-19 and/or CLL disease, we next identified the population cohort who tested PCR+ for SARS-CoV-2 outside the EHR system. Only OS could be assessed for this population. Gradually improving 30-days survival rates were demonstrated from periods 2 to 4 (93.9%, 94.5%, and 99.2%, respectively; no deaths were seen in time period 1, which started 16 September 2020 with mass testing; P < .002; pairwise log-rank, Figure 1D). When combining the 2 cohorts, 30-day OS rates gradually improved from periods 1 to 4 (88.0%, 89.6%, 93.3%, and 98.2%, respectively), with a significantly higher OS in the O BA.2 period compared with periods 1 to 3 (P ≤ .0077; pairwise log-rank, Figure 1E).
Limitations apply to this study; the size of the EHR patient population was limited, and patients with CLL testing positive for SARS-CoV-2 outside EHR test sites were only included in the population cohort. Thus, the improved outcome in the population cohort may reflect less severe CLL, less severe COVID-19, and/or less comorbidity.
Based on epidemiological data from South Africa,21 the incidence of SARS-CoV-2 seems decoupled from the incidences of hospitalization and death upon emergence of the Omicron variant, while previous vaccination seems to protect less against infection with the Omicron variant of SARS-CoV-2.22 This study indicate that Omicron sublineage BA.2 pose a similar risk of fatal COVID-19 only for patients with impaired immune function due to CLL and a close hospital contact either due to CLL or COVID-19,3,18 with an estimated 30-day OS rate of 77%. It should be emphasized that patients in the population cohort may also have been hospitalized, but no data on this were accessible. The overall population of patients with CLL seems to have a much milder course of COVID-19 during the era of the Omicron variant, especially during BA.2 dominance, with a 30-day fatality rate <2%. Thus, patients >70 with CLL and 1 or more comorbidities and hospital contact due to CLL or COVID-19 should be considered for closer monitoring and preemptive antiviral therapy upon a positive SARS-CoV-2 test.
Acknowledgments
The Capital Region of Denmark, Center for Economy, provided data extracts from the EHR system.
This study was supported by a COVID-19 grant from the Ministry of Higher Education and Science (0238-00006B) and the Danish National Research Foundation (DNRF126) and by the Danish Cancer Society, and the EU funded CLL-CLUE for C.U.N. C.B. received funding from Weimann’s Legat. Funding support for this article was provided by the Ministry of Higher Education and Science, Denmark (0238-00006B), Danish Cancer Society, EU ERA-PERMED program (CLL-CLUE), Danish National Research Foundation (DNRF126).
Authorship
Contribution: S.R.O., C.U.N., C.B., and C.d.C.-B. developed the concept of the study; C.B., C.U.N., and C.d.C.-B. collected and curated data; C.B. performed statistical analyses; M.H. provided infectious disease and clinical perspectives and interpretation; C.U.N. wrote the first draft of the paper; and all authors contributed to and approved the final version
Conflict-of-interest disclosure: C.U.N. received research funding and/or consultancy fees outside this work from Abbvie, Janssen, AstraZeneca, Beigene, Roche, CSL Behring, Takeda, and Octapharma. C.B. received consultancy fess outside of this work from AstraZeneca. The remaining authors declare no competing financial interests.
Correspondence: Carsten U. Niemann, Department of Hematology, Rigshospitalet, Copenhagen University Hospital, Building 5074, Blegdamsvej 9, DK-2100 Copenhagen Ø, Denmark; e-mail: carsten.utoft.niemann@regionh.dk.
Send data sharing requests via e-mail to the corresponding author.
There is a Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
REFERENCES
Author notes
S.R.O. and C.B. are joint senior authors.