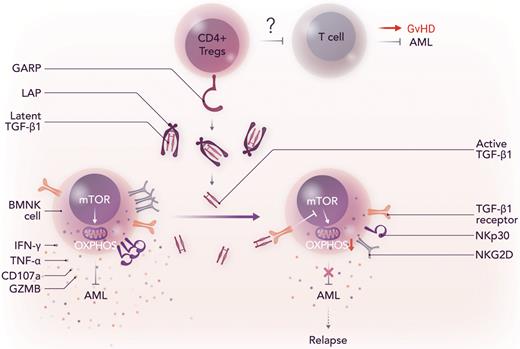
In this issue of Blood, Wang et al1 demonstrate that glycoprotein A repetitions predominant (GARP)–mediated activation of TGF-β1 by regulatory T cells (Tregs) downregulates the effector functions of natural killer (NK) cells in the bone marrow of patients with acute myeloid leukemia (AML) with early relapse after allogeneic hematopoietic cell transplantation (allo-HCT), and that pharmacologic blockade of TGF-β1 signaling using galunisertib or anti-TGF-β1 antibodies can restore the killer instinct of human NK cells.
Disease relapse remains the main cause of death after allo-HCT for AML.2,3 Although donor lymphocyte infusion (DLI; primarily T cells) has been used to treat AML relapse, the survival benefit for patients with relapsed AML by DLI is far from satisfactory with a 2-year overall survival (OS) of 25% and a 5-year OS of 15%.4 In addition, DLI is associated with a risk of graft-versus-host disease (GVHD), a leading cause of non-relapse mortality after DLI or allo-HCT.3,4 Thus, finding a means to prevent and treat AML relapse without inducing GVHD is an unmet medical need.
The current study proposes that the restoration of NK cell functions by targeting TGF-β1 may control disease relapse. NK cells are the first lymphocytes to reconstitute after allo-HCT and their successful recovery is associated with protection against AML relapse.5 In contrast to T cells, NK cells play a regulatory role in GVHD in addition to their potent anti-leukemia effect in the allogeneic setting.6 Thus, a number of NK cell–based immunotherapies have been investigated including a phase I clinical trial with donor memory-like NK cells for patients with relapsed AML after allo-HCT.7,8 Nonetheless, NK cell immunotherapies have been disappointing likely owing to impairment of NK cell cytotoxicity, thereby allowing AML cells to escape from immunologic destruction. In addition, the killer activity of NK cells derived from the donor graft against host AML has not been definitively demonstrated in relapsed AML after allo-HCT. Do NK cells, reconstituted after allo-HCT, manifest an active anti-leukemia effect? What causes NK cell dysfunction after allo-HCT? Can we develop therapeutic strategies to restore the cytotoxicity of NK cells against relapsed AML? If so, we may limit the need for expensive and labor-intensive ex vivo cellular manipulations involved in NK cell–based immunotherapies for hematologic malignancies and even for solid tumors (eg, adoptive transfer of autologous or allogeneic NK cells, memory-like NK cells, CAR NK cells, etc).
The study by Wang et al identifies the GARP-TGF-β1 pathway between Tregs and NK cells in the bone marrow of patients with AML as the major player in the loss of NK cell cytotoxicity against AML. The authors first showed that the levels of active TGF-β1 were significantly increased in the bone marrow of patients with relapsed AML than those without relapse even though the total amounts of TGF-β1 (both latent and active) in the bone marrow were comparable between the 2 groups. These findings lead them to the question of whether or not the increased levels of active TGF-β1 disarmed NK cells in the bone marrow (BMNK cells). They found that active TGF-β1 impaired the effector functions of BMNK cells by reducing the expression of IFN-γ, TNF-α, CD107a, GZMB, NKp30, and NKG2D and suppressing mTOR activity and mitochondrial respiration (OXPHOS) (see figure). Next, the authors determined the role of GARP that has been known to activate latent TGF-β1 in the context of AML. GARP + CD4 + T cells (FOXP3 + Tregs) were significantly more abundant in the bone marrow of patients with relapse than those without relapse. In addition, cytotoxicity of BMNK cells was significantly reduced when BMNK cells were pretreated with latent TGF-β1 in the presence of GARP + CD4 + T cells. Consistent with this, BMNK cells purified from patients with AML with relapse had significantly lower anti-tumor activities than those from patients without relapse. Importantly, the authors demonstrated that TGF-β1 inhibitors, such as galunisertib and anti-TGF-β1 antibodies, could restore the anti-leukemia effector functions of BMNK cells. Thus, the current study by Wang et al urges us to pay attention to the GARP-TGF-β1 pathway as a potential therapeutic target to prevent and treat AML relapse, thereby improving the survival of patients after allo-HCT or NK cell–based immunotherapies.
GARP-mediated activation of TGF-β1 by Tregs induces NK cell dysfunction after allo-HCT, which results in disease relapse. LAP, latency associated peptide, which prevents the binding of TGF-β1 to its receptor; OXPHOS, oxidative phosphorylation; mTOR, mammalian target of rapamycin. Professional illustration by Somersault18:24.
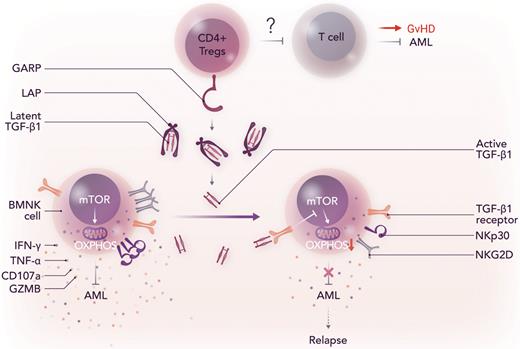
GARP-mediated activation of TGF-β1 by Tregs induces NK cell dysfunction after allo-HCT, which results in disease relapse. LAP, latency associated peptide, which prevents the binding of TGF-β1 to its receptor; OXPHOS, oxidative phosphorylation; mTOR, mammalian target of rapamycin. Professional illustration by Somersault18:24.
It is, nonetheless, also important to acknowledge that there are a few gaps that need to be further studied. Their previous report suggests that FBP1 is a key component in TGF-β1 signaling–mediated NK cell dysfunction in lung cancer.9 Does FBP-1–induced inhibition of glycolysis also play a role in AML relapse? Can FBP-1 inhibitors effectively control AML relapse by restoring NK cell function after allo-HCT? Because TGF-β1 signaling suppresses alloreactive T cells as well, it would be important to examine the status of allogeneic T cells in the bone marrow of patients with relapsed AML. Because TGF-β1 contributes to Treg differentiation and functions, targeting TGF-β1 may negatively affect the ability of Tregs to suppress alloreactive T cells, thereby increasing GVHD (see figure). In contrast, cytotoxicity of donor NK cells against GVHD-causing alloreactive T cells after allo-HCT is associated with GVHD reduction. Thus, it remains to be determined if targeting TGF-β1 signaling after allo-HCT can cause T cell–mediated GVHD.
Overall, the current study provides key insights into the role of NK cells in AML relapse and NK cell–based immunotherapies such as adoptive transfer of autologous or allogeneic NK cells, memory-like NK cells, and CAR NK cells, thereby having a transformative impact on the field of transplant immunology, with great potential to improve the outcome of patients undergoing allo-HCT and NK cell immunotherapies. The current study also supports further exploration of pharmacologic inhibition of the GARP-TGF-β1 pathway and its downstream signaling in patients with relapsed AML using small molecule inhibitors and anti-TGF-β1 antibodies. Fortunately, these 2 agents have already been developed and are in clinical trials for a broad range of solid tumors, including breast cancer, non–small cell lung cancer, colorectal cancer, prostate cancer, melanoma, pancreatic cancer, hepatocellular carcinoma, and glioblastoma.10
Conflict-of-interest disclosure: The authors declare no competing financial interests.