Key Points
Relapses are frequent after vemurafenib monotherapy, but retreatment with vemurafenib can induce high response rates in HCL.
Vemurafenib retreatment is safe with no additional observed adverse events.
Abstract
Vemurafenib, an oral BRAF inhibitor, has demonstrated high response rates in relapsed/refractory (R/R) hairy cell leukemia (HCL). However, little is known about long-term outcomes and response to retreatment. Herein, we report the results of 36 patients with R/R HCL treated with vemurafenib from the United States arm of the phase 2 clinical trial (NCT01711632). The best overall response rate was 86%, including 33% complete response (CR) and 53% partial response (PR). After a median follow-up of 40 months, 21 of 31 responders (68%) experienced relapse with a median relapse-free survival (RFS) of 19 months (range, 12.5-53.9 months). There was no significant difference in the RFS for patients with CR vs PR. Fourteen of 21 (67%) relapsed patients were retreated with vemurafenib, with 86% achieving complete hematologic response. Two patients acquired resistance to vemurafenib with the emergence of new KRAS and CDKN2A mutations, respectively. Six of 12 (50%) responders to vemurafenib retreatment experienced another relapse with a median RFS of 12.7 months. Overall survival (OS) was 82% at 4 years, with a significantly shorter OS in patients who relapsed within 1 year of initial treatment with vemurafenib. Higher cumulative doses or a longer duration of treatment did not lengthen the durability of response. All adverse events in the retreatment cohort were grade 1/2 except for 1 case of a grade 3 rash and 1 grade 3 fever/pneumonia. Our data suggest that vemurafenib retreatment is a safe and effective option for patients with R/R HCL.
Introduction
Hairy cell leukemia (HCL) is a rare chronic B-cell lymphoproliferative disorder characterized by a high prevalence of BRAF V600E mutation.1,2 Purine nucleoside analogs can achieve an overall response rate (ORR) of 90% to 100% and complete responses (CR) of 80% to 95% and remain the mainstay of first-line treatment in HCL.3,4 However, approximately 30% to 40% of patients will experience recurrent disease, and relapse-free survival (RFS) rates decrease with repeated courses of purine analog-based treatments with cumulative myelotoxicity and immune suppression.5,6
The genetic basis of HCL was first uncovered a decade ago when Tiacci and colleagues reported that BRAF V600E is a key mutation in HCL,7 a finding that was further validated by subsequent studies.8,9 Based on these findings, we conducted a multicenter phase 2 clinical trial in the United States, evaluating the efficacy and safety of vemurafenib, an oral BRAF inhibitor, in patients with relapsed/refractory (R/R) HCL. We previously published the initial outcome of 26 US patients together with the Italian study conducted by Tiacci and colleagues.7 Vemurafenib monotherapy induced 96% to 100% ORR and 35% to 42% CR rates in both studies. With a median follow-up duration of 11.7 months, we observed relapses in 29% of the patients, but the long-term clinical outcome and response or acquired resistance to vemurafenib retreatment have not been previously reported. Herein, we report the long-term follow-up data of the entire patient cohort in the completed US clinical trial (NCT01711632), including the ORR, RFS, clinical factors associated with improved survival, as well as outcomes after retreatment with vemurafenib or alternative agents.
Methods
Patients
The US trial (NCT01711632) was a phase 2 single-arm multicenter study that enrolled patients from 6 different sites between January 2013 and June 2016. Since the initial study report,7 we have enrolled 10 additional patients and herein report the outcome of a total of 36 patients treated in the study.
All patients tested positive for the presence of BRAF V600E mutation and met 1 of the following criteria: refractoriness to a purine analog evidenced by no response or relapse within 1 year of therapy, early relapse (between 1 and 2 years) following the initial course of purine analog, or ≥2 relapses that occurred >2 years after ≥3 courses of a purine analog. Eligibility criteria also required the presence of ≥1 cytopenias defined as hemoglobin ≤10 g/dL, platelet count ≤100 000/mm3, or absolute neutrophil count (ANC) ≤1000 per mm3. The clinical study was carried out in compliance with the Institutional Review Board protocol, in line with Good Clinical Practices and the ethical principles of the Helsinki Declaration. Informed consent form was reviewed and approved by the Institutional Review Board and signed by all participants at the beginning of the study.
Study design
The study design was previously published.7 During the initial treatment course, all patients received vemurafenib 960 mg orally twice daily continuously for a minimum of 3 months, with a provision to extend the treatment up to 6 months in case of residual disease. Dose adjustments were permitted for drug-related toxicity. On follow-up, patients with relapsed disease could be rechallenged with vemurafenib at doses ranging from 240 mg to 960 mg twice daily at the discretion of the primary oncologist based on prior tolerability. Relapse was defined as recurrent cytopenias that were low enough to meet the initial eligibility criteria after obtaining a partial or complete response to initial vemurafenib treatment. Retreatment with vemurafenib was continued until disease progression or occurrence of unacceptable toxicity, and treating physicians were allowed to decide the retreatment course duration based on clinical response. Adverse events (AEs) were graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events, version 4.03. Bone marrow (BM) assessments were performed at 1 and 3 months during the initial course of vemurafenib treatment. During subsequent follow-up periods, BM biopsy was only performed if clinically indicated and at the discretion of the treating physician. Next-generation DNA sequencing using Memorial Sloan Kettering Cancer Center-Integrated Mutation Profiling of Actionable Cancer Targets was performed post-hoc only on available DNA samples as previously described.10 Cancer cell fractions were calculated by adjusting the variant allele frequency for tumor purity and copy number.
Study objectives and definitions
The primary objective of the study was to report the overall survival (OS) and RFS after initial and subsequent courses of vemurafenib. Secondary objectives included assessment of the impact of clinical variables on OS and RFS, ORR, and safety. OS was calculated from the date of initiation of the first vemurafenib treatment to the date of death or the date of last follow-up. RFS was calculated from the end of initial vemurafenib treatment to the date of relapse or death. ORR was defined by the achievement of either complete response (CR), partial response (PR), or complete hematologic response (CHR). Definitions of CR, PR, and CHR have been previously described in the original trial.7 Complete resolution of splenomegaly and cytopenias along with a morphologic absence of peripheral and BM hairy cells qualified as a CR, whereas normalization of cell counts with ≥50% decrease in splenomegaly and BM hairy cells qualified for PR. Those with a resolution of cytopenias without a BM assessment were accounted as having a CHR. We also performed a nonprotocol planned exploratory landmark analysis of RFS in patients who achieved CR vs PR at 3 months, as well as a landmark analysis of OS in those who relapsed within 1 year of therapy initiation vs >1 year.
Statistical analysis
Patient characteristics are summarized by frequency (percentage). Fisher’s exact test was used to determine associations between baseline characteristics and the overall response. Kaplan-Meier method was employed to calculate OS and RFS. Survival differences between groups were analyzed using the log-rank test. The effects of patient and disease characteristics on OS were estimated by the univariate Cox proportional hazard model, with P < .05 being considered significant. The difference in median RFS between patients who received the first course of vemurafenib and patients who were retreated with vemurafenib was evaluated using the permutation approach. The treatment designation (ie, first and retreatment) for each permutation dataset was randomly assigned to every retreated patient. The P value was defined as the proportion of these permutation datasets with a larger difference in median RFS between 2 groups than the observed one. All statistical analyses were performed using R version 3.5.1 (R Foundation for Statistical Computing; Vienna, Austria).
Results
Baseline patient characteristics
Baseline patient characteristics of all 36 patients enrolled in the trial are summarized in Table 1. Patients were predominantly male (83%) with a median age of 59 years (range, 34-80 years). Patients received a median of 3 prior lines of therapy (range, 1-8), and the time from the initial diagnosis to the start of vemurafenib therapy varied widely from 1 to 33 years, with a median of 10.5 years.
Response duration and relapse after initial vemurafenib therapy
Of the 36 enrolled patients, 32 patients completed ≥4 weeks of treatment, whereas 4 patients stopped the medication in <4 weeks because of treatment-related toxicity (n = 2; grade 3 reversible photosensitivity and anaphylaxis), pneumonia (n = 1, considered by the investigator to be unrelated to the study drug), and patient request (n = 1).
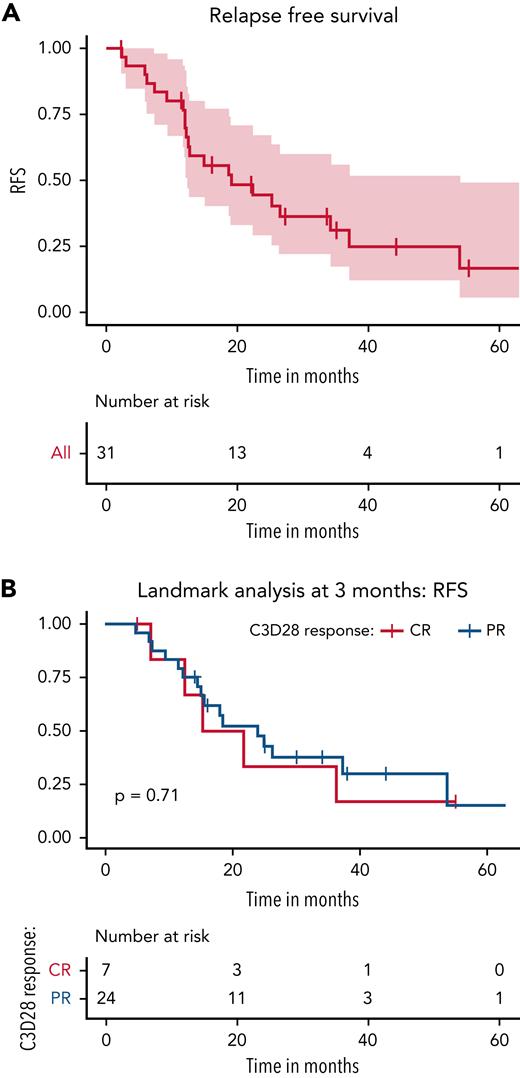
The best ORR in the entire cohort was 86% (31 out of 36 patients), including 33% CR (n = 12) and 53% PR (n = 19). Of the 32 patients who received ≥4 weeks of therapy, the median duration of vemurafenib treatment was 23.5 weeks (range, 7.5-30 weeks). With a median follow-up of 40 months (range, 1-87 months), 21 out of 31 patients who responded (68%) to initial vemurafenib treatment experienced a relapse with a median RFS of 19 months (95% confidence interval [CI], 12.5-53.9 months) (Figure 1A). Table 2 demonstrates the association of patient and treatment-related variables to RFS. None of the factors, including age, gender, number of prior lines of therapy, time since diagnosis to trial enrollment, history of splenectomy, or type of response to treatment, showed any significant association with RFS. A cumulative vemurafenib treatment dose >230 000 mg was associated with a significantly increased odds of relapse when compared with a lower cumulative dose of <150 000 mg (hazard ratio [HR], 7.75; 95% CI, 2.23-26.87; P = .0013). Similarly, there was a trend toward a higher relapse risk with a treatment duration of >90 days, although the association was not statistically significant. We performed a landmark analysis at 3 months (ie, after the protocol defined initial response assessment time point) and found no significant difference in the RFS between those who achieved PR vs CR (Figure 1B). The median RFS was 18.5 months (95% CI, 12.4 months to not reached) for those who achieved CR vs 23.9 months (95% CI, 14.9 months to not reached) for those who demonstrated PR.
Relapse free survival after 1st course of vemurafenib. (A) RFS after the first course of vemurafenib. A total of 21 out of 31 responders (68%) experienced a relapse after the initial course of vemurafenib with a median RFS of 19 months (95% CI, 12.5-53.9 months). (B) Landmark analysis of RFS in patients who achieved CR vs PR at 3 months. The median RFS was 18.5 months (95% CI, 12.4 months to not reached) for those who achieved CR vs 23.9 months (95% CI, 14.9 months to not reached) for those who demonstrated PR. This was not significantly different (P = .71).
Relapse free survival after 1st course of vemurafenib. (A) RFS after the first course of vemurafenib. A total of 21 out of 31 responders (68%) experienced a relapse after the initial course of vemurafenib with a median RFS of 19 months (95% CI, 12.5-53.9 months). (B) Landmark analysis of RFS in patients who achieved CR vs PR at 3 months. The median RFS was 18.5 months (95% CI, 12.4 months to not reached) for those who achieved CR vs 23.9 months (95% CI, 14.9 months to not reached) for those who demonstrated PR. This was not significantly different (P = .71).
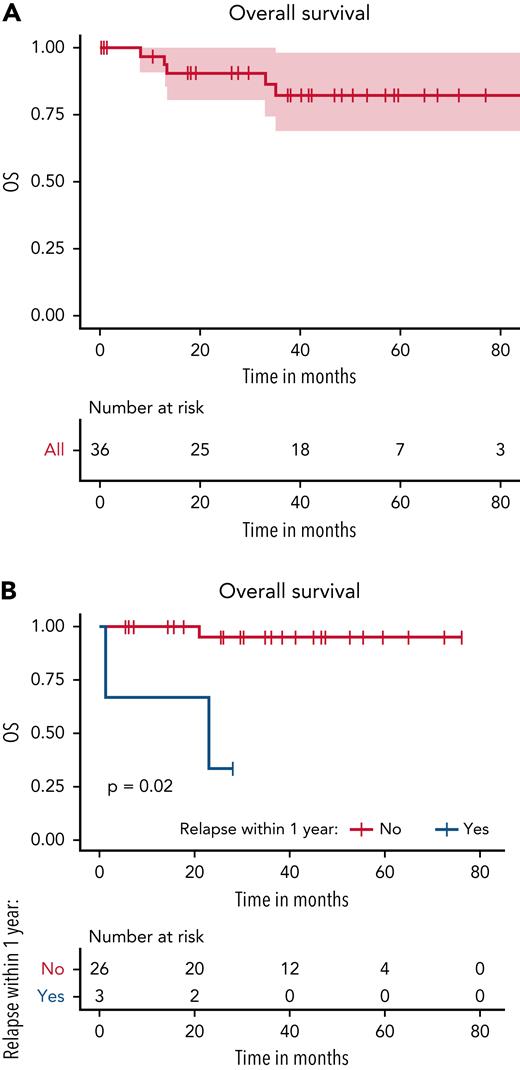
The 4-year OS for all patients was 82% (95% CI, 69%-98%) (Figure 2A). Only cumulative vemurafenib treatment dose <150 000 mg was associated with a significantly lower risk of relapse when compared with a dose >230 000 mg (Table 3), and the landmark analysis performed at 1 year revealed a significantly higher OS (P = .02) for patients who did not relapse within the first year of treatment (Figure 2B).
Overall survival. (A) OS for all patients at 4 years was 82% (95% CI, 69%-98%). (B) Landmark analysis after 1 year of starting vemurafenib therapy showed a significantly higher OS (P = .02) for patients who did not relapse within the first year of treatment.
Overall survival. (A) OS for all patients at 4 years was 82% (95% CI, 69%-98%). (B) Landmark analysis after 1 year of starting vemurafenib therapy showed a significantly higher OS (P = .02) for patients who did not relapse within the first year of treatment.
Subsequent treatments following relapse after initial vemurafenib therapy
Among the 21 patients with relapse, 14 patients (67%) received retreatment with vemurafenib; 6 patients (28.5%) received alternative treatments such as rituximab and cladribine combination (n = 2), pentostatin and rituximab (n = 1), moxetumomab pasudotox (n = 1), and ibrutinib (n = 2); and 1 patient was observed without any further treatment.
Among the 6 patients retreated with alternative regimens, response data are only available for 3 patients because they were treated off-study. One patient received 8 weeks of cladribine and rituximab, resulting in stable disease and a treatment-free survival of 26 months after completion of therapy. A second patient treated with ibrutinib achieved CHR after 15.5 weeks of therapy, and CHR was sustained for 13 months after the end of treatment and has been off all therapy for 18 months at last follow-up. A third patient received a 4-month course of pentostatin and rituximab, resulting in CR for ≥13 months at the time of the last follow-up.
Retreatment with vemurafenib
Of the 14 patients retreated with vemurafenib, starting doses of vemurafenib were 960 mg (n = 2), 720 mg (n = 1), 480 mg (n = 4), and 240 mg twice daily (n = 7). The starting dose at retreatment was either lower (n = 5) or the same as the last tolerated dose (n = 5) because of concerns about the recurrence of drug-related AEs experienced during the initial treatment, whereas four patients were treated with a higher than prior dose. In the study, patients were able to continue vemurafenib for any duration and received a median of 10 cycles of vemurafenib (range, 1-71 cycles) over a median duration of 8.5 months (range, 0.3-66.5 months). Seven of the 14 retreated patients successfully completed ≥10 cycles of vemurafenib. Figure 3 summarizes the overall clinical course of 14 vemurafenib-retreated patients.
The clinical course of 14 patients who were retreated with vemurafenib. Patient IDs 2 and 4 were found to have acquired resistance to vemurafenib via CDKN2A loss and activating KRAS mutations, respectively.
The clinical course of 14 patients who were retreated with vemurafenib. Patient IDs 2 and 4 were found to have acquired resistance to vemurafenib via CDKN2A loss and activating KRAS mutations, respectively.
Twelve of the 14 patients (86%) achieved at least CHR to retreatment with vemurafenib; 1 patient was refractory with development of acquired KRAS mutation as previously reported,7 and 1 patient discontinued vemurafenib in <4 weeks because of grade 2 photosensitivity and fatigue and was not evaluable for a response. Among the 12 patients who achieved CHR, 8 patients had post-treatment BM biopsy enabling further classification of response type; 1 patient achieved a CR, and 7 patients achieved a PR. The median time to hematologic recovery was 3.5 weeks (range, 1-12 weeks), 8 weeks (range, 2-12.5 weeks), and 8 weeks (range, 3.5-12 weeks) for platelets, ANC, and hemoglobin, respectively, as compared with 2, 4, and 8 weeks after initial treatment.7
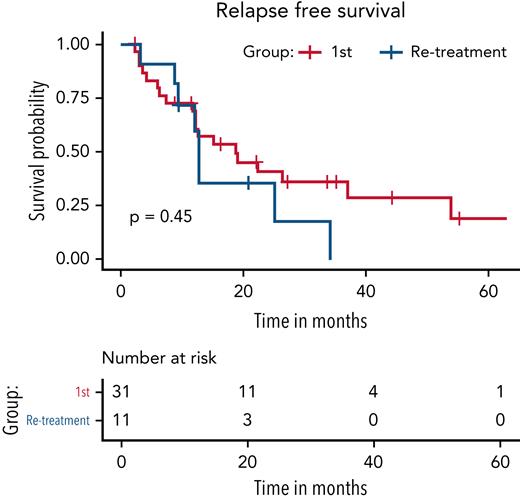
Six of the retreated patients experienced another relapse during the follow-up period. RFS was calculated for 11 out of 12 patients as 1 patient remained on treatment at the end of follow-up. Median RFS was 12.7 months (95% CI, 4.2 months to not reached), which was not significantly shorter than the RFS of 19 months (95% CI, 12.5-53.9 months) after the first course of vemurafenib (P = .47) (Figure 4). Of the 6 relapsed patients after vemurafenib retreatment, 2 patients were rechallenged with vemurafenib; 1 patient was treated with pentostatin; 2 patients were observed without further therapy; and 1 patient had missing data.
Comparison of RFS for patients after the first course of vemurafenib vs retreatment with vemurafenib. Median RFS was 12.7 months (95% CI, 4.2 months to not reached) for retreated cohort, which was not significantly shorter than the RFS of 19 months (95% CI, 12.5-53.9 months) after the first course of vemurafenib (P = .47).
Comparison of RFS for patients after the first course of vemurafenib vs retreatment with vemurafenib. Median RFS was 12.7 months (95% CI, 4.2 months to not reached) for retreated cohort, which was not significantly shorter than the RFS of 19 months (95% CI, 12.5-53.9 months) after the first course of vemurafenib (P = .47).
Among the 2 patients who received a third course of vemurafenib, 1 patient achieved a CHR at 8 weeks and discontinued therapy in 6 months. However, she experienced relapse within 3 months, necessitating reinitiation of therapy at a lower dose of 240 mg every other day (dose reduced because of previous AE) for a total of 21 months, including 3 months of combination therapy with rituximab plus vemurafenib. The other patient was initially treated with vemurafenib 240 mg twice daily and achieved CHR in 12.5 weeks but developed progressive disease after 21 months with lymphocytosis and increasing circulating hairy cells but with no cytopenia. The dose of vemurafenib was increased to 720 mg twice daily, but the disease continued to progress, and vemurafenib was discontinued at 37 months.
AEs
The side effect profile during vemurafenib retreatment was akin to the initial treatment and mainly included arthralgia (57%), rash (29%), photosensitivity (36%), and fatigue (29%). All AEs were grade 1 or 2, except for 1 case of grade 3 rash, which was reversible after treatment cessation, and another case of grade 3 fever/pneumonia requiring a brief treatment hold (supplemental Table 1 in the data supplement, available on the Blood Web site). One patient experienced cystoid macular edema requiring drug discontinuation with prompt improvement in ocular symptoms afterward. Cutaneous malignancies, including squamous cell carcinomas (SCCs) (n = 6) and basal cell carcinoma (BCC) (n = 1), were reported in a total of 7 patients. Four of these patients had a predisposing medical history, including SCC (n = 1), BCC (n = 1), BCC/melanoma in situ (n = 1), and actinic keratosis (n = 1). All patients underwent local excision and did not require early drug termination. However, during the course of retreatment, 1 patient developed tonsillar SCC, and another patient with a history of melanoma developed metastatic melanoma, and vemurafenib was discontinued in both. AEs led to dose reductions in 50% of the patients (n = 7). Similar to initial therapy, the most frequent reasons for dose reductions were arthralgias (n = 4), fatigue (n = 3), rash (n = 2), and photosensitivity (n = 3). Most patients had >1 overlapping reason for dose reduction. Supplemental Table 1 lists all AEs during the initial and retreatment courses.
Mutational analysis of vemurafenib-retreated patients
Serial peripheral blood and/or BM samples at the time of initial vemurafenib initiation and at vemurafenib retreatment were available for 3 out of 12 retreated patients. One patient was treated with vemurafenib 240 mg twice daily for the third relapse following initial vemurafenib treatment. He initially achieved CHR in 12.5 weeks but developed progressive disease on vemurafenib. In this patient, we observed the subclonal outgrowth of a CDKN2A frameshift mutation following vemurafenib retreatment, eventually leading to vemurafenib resistance (Figure 5A; supplemental Table 2). In the other 2 patients, we observed evidence of clonal evolution with acquired HIST1H1D, PCBP1, KEAP1, and NF1 mutations leading to clinical relapse after initial vemurafenib treatment, but both patients achieved a complete hematologic response to vemurafenib retreatment (Figure 5B-C; supplemental Table 2).
Mutations were found in patients at the time of vemurafenib retreatment and 1 case of acquired resistance to vemurafenib. (A-C) Fishplot representation of sequencing data collected from patients at the time of vemurafenib treatment initiation and subsequent vemurafenib retreatment. Each patient had BRAFV600E mutation at the start of treatment that was suppressed during treatment and reappeared at the beginning of retreatment with the emergence of new subclones and/or persistence of prior subclones. (A) Patient ID1 had an initial complete hematologic response to vemurafenib retreatment but stopped responding during retreatment and was noted to have acquired a CDKN2A mutation at the time of resistance. Patient ID2 and Patient ID3 had acquired (B) HIST1H1D and PCBP1 mutations and (C) KEAP1 and NF1 mutations at the time of vemurafenib retreatment, but both patients achieved a complete hematologic response.
Mutations were found in patients at the time of vemurafenib retreatment and 1 case of acquired resistance to vemurafenib. (A-C) Fishplot representation of sequencing data collected from patients at the time of vemurafenib treatment initiation and subsequent vemurafenib retreatment. Each patient had BRAFV600E mutation at the start of treatment that was suppressed during treatment and reappeared at the beginning of retreatment with the emergence of new subclones and/or persistence of prior subclones. (A) Patient ID1 had an initial complete hematologic response to vemurafenib retreatment but stopped responding during retreatment and was noted to have acquired a CDKN2A mutation at the time of resistance. Patient ID2 and Patient ID3 had acquired (B) HIST1H1D and PCBP1 mutations and (C) KEAP1 and NF1 mutations at the time of vemurafenib retreatment, but both patients achieved a complete hematologic response.
Discussion
Herein, we report the long-term outcomes of patients from the US phase 2 clinical trial of vemurafenib in R/R HCL (NCT01711632), now with a median follow-up of 40 months (range, 1-87 months). We focused analysis on the clinical course of patients who relapsed and were retreated with vemurafenib. We found that while relapses were frequent, all patients retained BRAF V600E mutation on relapse, and vemurafenib retreatment was highly effective with 86% ORR.
Among the 2 patients who developed resistance to vemurafenib, one was because of 2 activating KRAS mutations and the other because of acquired CDKN2A loss after a third vemurafenib treatment course. Recently, CDKN2A loss has been associated with vemurafenib resistance and was preclinically able to be reversed with the addition of the CDK4/6 inhibitor palbociclib.11,12 Loss of CDKN2A releases the negative regulation of cyclin D and CDK4, which in turn activates the MAPK pathway.13 These results reinforce the exquisite reliance of HCL on this pathway for survival.
In our study cohort, we found age, gender, splenectomy status, number of prior therapies, and type of response (CR vs PR) did not significantly impact the RFS. We observed a significant association between a higher cumulative dosage of vemurafenib (>230 000 mg) and the risk of relapse. However, this finding likely reflects the study design wherein patients with suboptimal response/residual disease after the initial disease assessment at 3 months were allowed to receive additional vemurafenib treatment for up to 3 additional months. In another study, depth of response (CR vs PR) and a higher starting dose of vemurafenib (≥480 mg twice daily) were significantly associated with the response duration.8 Similarly, the Italian phase 2 study of vemurafenib in R/R HCL reported that patients who achieved CR to vemurafenib had a longer remission duration.7 In contrast, we found no difference in the RFS among those with a PR or CR to initial therapy, which could be related to different duration of initial vemurafenib treatment and time point of BM assessment to determine response.
During the follow-up period, 50% of patients (6 out of 12) who responded to the second course of vemurafenib had another relapse at a median of 12.7 months since treatment cessation, which was not significantly shorter when compared with the median RFS of 19 months after initial course (P = .47). Dietrich and colleagues have previously reported a significant decline in the response duration after third and subsequent courses of vemurafenib.8 Although HCL generally carries a favorable long-term prognosis, relapse within 1 year of treatment with BRAF inhibitor may portend a worse prognosis as depicted in our study but needs further validation in future studies.
The appropriate dosing and duration of vemurafenib therapy also require further elucidation. We used a dose of 960 mg twice daily for initial treatment, whereas doses ranging from 240 mg to 960 mg twice daily were used for retreatment depending on prior tolerability. Dose reductions were a frequent event in our study, with 61% of patients (22 of 36) from the initial cohort and 50% (7 of 14) from the retreatment cohort requiring dosage reduction. Another study of 17 patients who received vemurafenib at 240 mg twice daily dosing demonstrated similar rates and duration of response as the higher dosing with no discernable differences in recovery of blood count kinetics for patients who received low doses of vemurafenib (≤240 mg vs >240 mg).9 In our cohort, the median time for platelet (3.5 vs 2 weeks) and ANC recovery (8 vs 4 weeks) after retreatment was numerically longer compared with the initial course, but it is unclear whether this is related to delayed resensitization to MAPK pathway inhibition upon rechallenge or to dose effect.
Twice daily dosing of 960 mg has been extrapolated from dose expansion trials in melanoma on the basis of dose-limiting toxicities.14 This study also demonstrated that a minimum dose of 240 mg twice daily was necessary to achieve a pharmacodynamic effect in melanoma.14 However, we have observed a prompt and complete hematological response with a dose of as low as 240 mg daily used in 2 patients as well in the case reported by Bailleux and colleagues.15 This points to fundamental genomic differences in advanced melanoma, which is known to harbor activating BRAF mutation in 40% to 60% of cases and has a much more intricate genomic landscape16-18 as compared with HCL, wherein BRAF mutation seems to be a universal genetic trigger.1 Thus, starting at a lower dose and escalating as needed for an adequate response may be an alternative strategy to improve the toxicity profile in this indolent and chronic leukemia. No positive correlation has emerged between the duration of treatment and response duration in studies so far, including ours, suggesting that intermittent dosing for the shortest duration required to achieve a response may be pursued.7,8 Intermittent vs continuous dosing strategy has recently been shown to be equally efficacious in patients with advanced melanoma, with intermittent dosing being associated with lesser resistance and toxicity.19-21
Outcomes in patients who receive alternative regimens for treatment of relapse after vemurafenib remain undefined. Although limited by numbers, all 4 patients who received alternative regimens in our study (3 after initial relapse and 1 patient after second-time relapse) achieved durable remission ranging from 13 to 26 months. All of these patients had been previously exposed to the agents they received (including cladribine + rituximab, pentostatin + rituximab, pentostatin alone, and ibrutinib) and continued to retain sensitivity. This suggests that BRAF inhibition may not impact the responsiveness to other agents. Side effect profiles on retreatment were similar to that after the initial therapy as well as other reports.8,9 However, the frequency of grade 2/3 arthralgias (31% vs 14%) and rash (56% vs 29%) were less common in the retreatment cohort, presumably because of lower starting doses. Cutaneous malignancies (SCC and BCC) developed in 20% of the patients, which is congruent with prior studies in HCL as well as in melanoma.8,9,22
Thus, vemurafenib has emerged as a viable treatment option for R/R HCL, particularly in patients with myelosuppression or active infection.23,24 Our data indicate that clinical resistance to vemurafenib is infrequent, but relapses and incomplete responses are common with vemurafenib monotherapy. This high risk of relapse stems, in part, from the inability of vemurafenib to completely inhibit ERK1/2 phosphorylation downstream of BRAF. The Italian trial by Tiacci and colleagues has previously demonstrated the persistence of phospho-ERK expression in residual HCL cells in the marrow after prolonged treatment with vemurafenib.7,25,26 Another potential mechanism of relapse is tumor escape from BRAF inhibition as previously described in melanoma because of epigenetic changes,14 plasticities of signaling pathways that allow for phenotype switching,15,16 and quiescent stem-like cells that maintain tumor dormancy and drive future relapses.17
Several strategies to overcome this challenge are currently under investigation. In a phase 2 study by Tiacci and colleagues, the combination of vemurafenib and rituximab in patients with R/R HCL after treatment with nucleoside analogs was shown to produce a faster and deeper response compared with vemurafenib alone.27 The ORR was 96%, including 5 patients who were previously refractory to rituximab and 7 who had relapsed following a BRAF inhibitor. Interestingly, minimal residual disease (MRD) was absent in the BM of 17 of 27 patients (63%) and persisted after cessation of therapy in 16 of 17 patients. In comparison, residual BM disease was a constant feature of all 26 patients in the Italian trial of vemurafenib monotherapy.7 Similarly, vemurafenib in combination with obinutuzumab is being evaluated as a frontline therapy in patients with previously untreated HCL (NCT03410875), and the preliminary data shows high CR and MRD negativity rates, supporting this combination approach.28 Combination of BRAF and MEK inhibition has been proven to significantly improve the progression-free survival in melanoma and has replaced BRAF inhibitor monotherapy in clinical practice.29 However, a study of dabrafenib in combination with the oral MEK inhibitor, trametinib, did not deliver significantly better outcomes than vemurafenib alone in heavily pretreated R/R HCL patients with 78% ORR and 49% CR.30 A clinical trial evaluating other BRAF and MEK inhibitors such as encorafenib and binimetinib for R/R HCL is currently underway (NCT04324112).
Conclusions
This prospective multicentric study confirms that vemurafenib monotherapy is a highly effective option, regardless of the dosage or duration of therapy, in patients with R/R HCL with a favorable safety profile. Vemurafenib retreatment can achieve high response rates, and acquired resistance is rare. However, remission durations are shorter with each subsequent relapse, and a combination with anti-CD20 antibodies may shorten the duration of vemurafenib treatment and prolong remission durations in R/R HCL.
Acknowledgments
This work was supported by the NIH/NCI 1P50 254838-01 (O.A.-W. and M.S.T.) and the Hairy Cell Leukemia Foundation/Leukemia & Lymphoma Society Synergistic Team Award.
Authorship
Contribution: J.H.P. designed the study and wrote the manuscript; S.H., J.-O.L., and J.H.P. collected the data, conducted the analysis, and wrote the manuscript; R.M.S., A.S., J.K.A., M.R.G., K.R.R., O.A.-W., M.S.T., and J.H.P. enrolled patients to the clinical trial and took care of the patients; M.S. and S.V. participated in data collection; A.D. conducted the statistical analysis; S.M., J.T., and O.A.-W. conducted the genomic analysis; and all the authors reviewed and approved the manuscript.
Conflict-of-interest disclosure: R.M.S. has received advisory or consulting fees from AbbVie, Actinium, Agios, Arog, Astellas, Biolinerx, Celgene, Daiichi-Sankyo, Elevate, Gemoab, Janssen, Jazz, Macrogenics, Novartis, OncoNova, Syndax, Syntrix, Syros, Takeda, Trovagene, BergenBio, Foghorn Therapeutics, GSK, Aprea, Innate, Amgen, CTI Pharmaceuticals, BMS, and Boston Pharmaceuticals. A.S. serves as a consultant for AstraZeneca and Innate Pharmaceuticals. J.K.A. has received advisory or consulting fees from AbbVie, Amgen, Astellas, Daiichi Sankyo, Kura Oncology, Stemline, Syros, and Theradex; research funding at an institutional level for the conduct of trials from ALX Oncology, Amgen, Aptos, Astellas, Aprea, BioSight, BMS, Boehringer Ingelheim, Celgene, FujiFilm, Immunogen, Kartos, Kura Oncology, Loxo, and Takeda; reimbursement for travel from BioSight; and serves on a data monitoring committee for Glycomimetics. M.R.G. has received advisory or consulting fees from Pharmacyclics, AstraZeneca, and Sereno/Merck and serves on a data safety monitoring committee for Ascerta. J.T. has received honorarium from Karyopharm Therapeutics. O.A.-W. has served as a consultant for H3B Biomedicine, Foundation Medicine Inc, Merck, Prelude Therapeutics, and Janssen, and is on the Scientific Advisory Board of Envisagenics Inc., AIChemy, Harmonic Discovery Inc., and Pfizer Boulder and has received prior research funding from H3B Biomedicine, LOXO Oncology, and Nurix Therapeutics unrelated to the current manuscript. M.S.T. has received research funding from Abbvie, Cllerant, Orsenix, ADC Therapeutics, Biosight, Glycomimetics, Rafael Pharmaceuticals, and Amgen; has received advisory fees from AbbVie, BioLineRx, Daiichi-Sankyo, Orsenix, KAHR, Rigel, Nohla, Δ Fly Pharma, Tetraphase, Oncolyze, Jazz Pharma, Roche, Biosight, Novartis, Innate Pharmaceuticals, Kura, and Syros Pharmaceuticals; has consulted for Innate Phamaceuticals; and receives royalties from UpToDate. J.H.P. has received advisory or consulting fees from Allogene, Amgen, Artiva, Autolus, BMS, Curocel, Incyte, InnatePharma, Kite Pharma, Kura Oncology, Minerva, Novartis, Pfizer, PrecisionBio, and Servier and serves on a data monitoring committee for Affyimmune, BrightPharma, and Intellia. M.R.G. received research funding from Hairy Cell Leukemia Foundation and serves as Chair of Scientific Review Committee for Hairy Cell Leukemia Foundation (uncompensated). The remaining authors declare no competing financial interests.
Correspondence: Jae H. Park, Leukemia Service, Department of Medicine, Memorial Sloan-Kettering Cancer Center, 530 East 74th St, New York, NY 10021; e-mail: parkj6@mskcc.org.
References
Author notes
The data generated in this study are available upon request from the corresponding author.
The online version of this article contains a data supplement.
There is a Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement”in accordance with 18 USC section 1734.
S.H. and J.-O.L. contributed equally to this study.