Key Points
HLA-Fc with A2 (A2Fc) and B7 (B7Fc) antigens triggered antigen-specific and Fc-dependent killing of cognate B-cell hybridomas in vitro.
A2Fc treatment abolished outgrowth of A2-specific B-cell hybridomas in vivo and mitigated anti-A2−mediated platelet refractoriness.
Abstract
Platelet transfusion and transplantation of allogeneic stem cells and solid organs are life-saving therapies. Unwanted alloantibodies to nonself human leukocyte antigens (HLAs) on donor cells increase the immunological barrier to these therapies and are important causes of platelet transfusion refractoriness and graft rejection. Although the specificities of anti-HLA antibodies can be determined at the allelic level, traditional treatments for antibody-mediated rejection nonselectively suppress humoral immunity and are not universally successful. We designed HLA-Fc fusion proteins with a bivalent targeting module derived from extracellular domains of HLA and an Fc effector module from mouse IgG2a. We found that HLA-Fc with A2 (A2Fc) and B7 (B7Fc) antigens lowered HLA-A2− and HLA-B7−specific reactivities, respectively, in sera from HLA-sensitized patients. A2Fc and B7Fc bound to B-cell hybridomas bearing surface immunoglobulins with cognate specificities and triggered antigen-specific and Fc-dependent cytotoxicity in vitro. In immunodeficient mice carrying HLA-A2–specific hybridoma cells, A2Fc treatment lowered circulating anti−HLA-A2 levels, abolished the outgrowth of hybridoma cells, and prolonged survival compared with control groups. In an in vivo anti-HLA-A2−mediated platelet transfusion refractoriness model, A2Fc treatment mitigated refractoriness. These results support HLA-Fc being a novel strategy for antigen-specific humoral suppression to improve transfusion and transplantation outcomes. With the long-term goal of targeting HLA-specific memory B cells for desensitization, further studies of HLA-Fc’s efficacy in immune-competent animal models are warranted.
Introduction
Few therapies exist to selectively remove or suppress unwanted antibodies after alloimmunization. Such therapies would be useful in the setting of allogeneic transfusion or transplantation, where alloantibodies to mismatched donor antigens constitute immunological barriers to donor access and impair therapeutic outcomes. In the era of potent agents for T-cell suppression, donor-specific alloantibodies (DSAs) have emerged as a leading cause of refractoriness to platelet transfusion,1 engraftment failure after mismatched hematopoietic stem cell transplantation (HSCT),2-4 and rejection of transplanted solid organs.5,6 In the absence of effective interventions, thrombocytopenic patients who fail to respond to platelet transfusion may suffer from fatal bleeds,7 and transplant recipients may experience shortened graft survival and increased transplant-related mortality.2,8,9
HLA molecules are the primary target of alloantibodies in cases of platelet transfusion refractoriness and graft rejection. HLA molecules are highly immunogenic and widely expressed on platelets,10,11 stem cells,12,13 and solid organs. Because of the HLA diversity in the human population,14 random recipient-donor pairs are frequently HLA mismatched and at risk for alloimmunization and rejection. Many patients are also sensitized to nonself HLA due to prior exposures, which can impact the success of subsequent platelet transfusion or transplantation. Consequently, preformed alloantibodies to high-prevalence antigens such as HLA-A2 (denoted throughout as A2) and HLA-B7 (denoted throughout as B7) (48% and 21% of the donor population, respectively) severely limit the pool of suitable donors. When serologically compatible donors are unavailable, incompatible donors may be considered. However, crossing the DSA barrier carries a high risk of ineffective platelet transfusion or graft failure. Mechanistically, DSAs mediate rapid clearance of platelets by macrophages15 and cause graft injury via complement activation or complement-independent processes.16
Traditional therapies for alloantibodies take the form of global suppression of humoral immunity, often using plasma exchange, pan−B-cell depletion, or the inhibition of plasma cells.17 These approaches have demonstrated variable effects in clinical trials and have been associated with severe infections. Although clinical laboratories can detect and identify anti-HLA antibodies with allelic precision,18-21 no treatment is available to selectively eliminate DSAs. Here, we describe HLA-Fc fusion proteins that can target antigen-specific antibody-producing cells for complement-dependent cytotoxicity in vitro. These fusion proteins can deplete target cells in a murine model, reduce circulating anti-HLA antibodies, and mitigate antibody-mediated platelet clearance. These findings support HLA-Fc as a novel strategy for selective humoral desensitization to improve transfusion and transplantation outcomes.
Methods
Human serum specimens and platelet donors
Seven serum specimens from patients sensitized to A2, B7, or both were used for this study. These sera were remaining specimens after routine clinical testing and were anonymized at the time of collection; subsequent experiments were determined to be nonhuman subject research by the Human Research Protection Office (HRPO) at Washington University in St Louis School of Medicine (institutional review board identification no. 202107106). For platelet transfusion experiments, informed consent was obtained from HLA-typed adult volunteers without bleeding disorders, and peripheral blood was collected and used following a protocol approved by the HRPO (institutional review board identification no. 202104132).
Mice
C57BL/6J (stock no. 000664; abbreviated “B6”), B6.129S-Rag2tm1FwaCd47tm1FplIl2rgtm1wjl/J (stock no. 025730; abbreviated “TKO”) and C57BL/6-Mcph1Tg(HLA-A2.1)1Enge/J (stock no. 003475; “A2 transgenic”) mice were obtained from the Jackson Laboratory. All mice were maintained in our specific pathogen-free animal facility. Experiments used mice of both sexes aged 8 to 24 weeks. Sex-matched littermates were used in individual experiments whenever possible. All animal experiments were approved by the Institutional Animal Care and Use Committee of Washington University in St Louis (protocol identification no. 20-0057).
Cell lines
Expi293F cells (Gibco) were grown in suspension in Expi293 Expression Medium (Gibco) supplemented with antibiotics in a shaking incubator at 37°C until transfection for HLA-Fc production. PA2.1 (producing anti-A2; HB-117) and BB7.1 (producing anti-B7; HB-56) murine B-cell hybridomas were obtained from the American Type Culture Collection (ATCC) and cultured in Iscove modified Dulbecco medium (IMDM) supplemented with hypoxanthine and thymidine (ATCC), fetal bovine serum (10%), and antibiotics (penicillin and streptomycin) at 37°C.
Generation and purification of recombinant HLA-Fc proteins
Sequences encoding the single-chain trimers (SCT) for HLA-A∗02:01 (A2)22,23 and HLA-B∗07:02 (B7) were cloned into the pFUSE-mIgG2a-Fc1 vector (InvivoGen) for the expression of A2Fc and B7Fc, respectively. The loss-of-function LALAPG mutations in Fc were introduced by site-directed mutagenesis to eliminate complement- and Fc-γ−dependent cytotoxicity24 to create the A2Fc-LALAPG as a control protein. Plasmids were transfected into Expi293F cells using Hype-5 transfection reagent (OZ Biosciences). Supernatants were harvested on day 5, and HLA-Fc fusion proteins were purified by standard protein A chromatography. Proteins were exchanged to 1× phosphate-buffered saline (PBS) at 1 mg/mL, snap-frozen, and stored at −80°C.
Single-antigen bead assay
Single-antigen bead (SAB) assay was performed using the LABScreen SAB kit (Class I; One Lambda) on the Luminex 200 instrument (Luminex Corporation) as described previously.18
Complement-dependent cytotoxicity assay
For the complement-dependent cytotoxicity (CDC) assay, B-cell hybridomas were incubated with HLA-Fc at the indicated concentrations in rabbit serum at room temperature for 3 hours. Washed cells were stained with 7-aminoactinomycin D (7-AAD) to assess viability by flow cytometry, and surviving cells (7-AAD−) in treated and untreated samples were quantified.
In vivo B-cell hybridoma model and HLA-Fc treatment
TKO mice received 1 million PA2.1 cells via IV injection immediately followed by intraperitoneal injection of 0.4 mg of A2Fc or A2Fc-LALAPG or no treatment. Peripheral blood was collected before cell transfer (prebleed) and weekly for up to 7 weeks in cases in which mice remained alive. Sera were crossmatched against A2 transgenic splenocytes to determine anti-A2 levels, reported as median channel shift (MCS) relative to the prebleed specimen. Upon sacrifice because of morbidity or at the end of a follow-up period of up to 120 days, organs were grossly examined and collected for histologic examination.
Platelet transfusion refractoriness model
TKO mice carrying PA2.1 cells with or without A2Fc treatment received 0.2 mL A2+ human platelet−rich plasma via IV infusion at 2 weeks after PA2.1 cell injection. TKO mice that did not receive PA2.1 cells served as controls. Peripheral blood was collected at 10, 60, and 120 minutes after transfusion. Human and murine platelet counts were quantified by immunostaining and flow cytometry as detailed in the supplemental Methods, available on the Blood website.
Statistical analysis
All analyses were performed using Prism version 9.2.0 (GraphPad Software, LLC) as detailed in the supplemental Methods.
Results
Design and production of HLA-Fc proteins
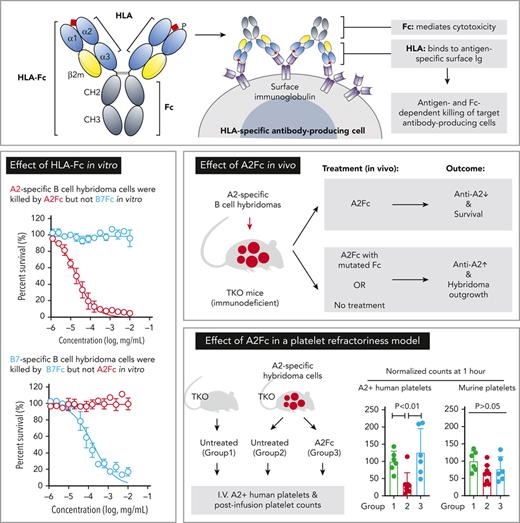
The extracellular domains of class I HLA include 2 membrane-distal, peptide-presenting domains (α1 and α2) and 2 membrane-proximal immunoglobulin-folds (α3- and β2-microglobulin).25 The overall dimensions are comparable to the antigen-binding fragment (Fab) of an antibody.25,26 We hypothesized that class I HLA could be coupled with the Fc region of IgG to form an antibody-like homodimer with bilateral symmetry (Figure 1A). We expected that such fusion proteins would bind to the surface immunoglobulin of anti-HLA antibody−producing cells and trigger cytotoxicity via Fc-dependent effector functions (Figure 1B).
Design and production of human leukocyte antigen (HLA)-Fc fusion proteins. (A) Schematic representation of HLA-Fc fusion proteins. The N-terminus of the fusion protein is a single-chain trimer (SCT) of a 9-mer peptide (red), β2-microglobulin (β2m, yellow), and extracellular domains of HLA class I (α1-α3, blue). The C-terminus consists of the constant domains 2 and 3 of the IgG heavy chain (CH2 and CH3, gray) or a fragment crystallizable (Fc). Disulfide bonds at the hinge region of Fc allow the homodimerization of HLA-Fc with bilateral symmetry. (B) The proposed mechanism of action for HLA-Fc. The HLA portion binds to cognate surface immunoglobulin (Ig, purple) and guides the fusion protein to corresponding antibody-producing cells; the Fc portion mediates cytotoxicity to enable target cell killing. (C) Components of HLA-Fc proteins used in the study. All variants start with a signal sequence, followed by a 9-mer peptide, flexible linker #1, human β2m (hβ2m), flexible linker #2, extracellular domains of the indicated HLA allele, and Fc of mouse IgG2a. The broken lines between linker #1 and the α2 domain represent disulfide bonds that secure the 9-mer peptides within the peptide-presenting domains (α1-α2). The A2Fc−LALAPG mutant contains 3 point mutations within the Fc portion (arrowheads). (D) Characterization of HLA-Fc proteins by polyacrylamide gel electrophoresis and Coomassie stain. HLA-Fc variants were purified with protein A from the supernatant of transiently transfected Expi293 cells and analyzed in nonreduced and reduced conditions.
Design and production of human leukocyte antigen (HLA)-Fc fusion proteins. (A) Schematic representation of HLA-Fc fusion proteins. The N-terminus of the fusion protein is a single-chain trimer (SCT) of a 9-mer peptide (red), β2-microglobulin (β2m, yellow), and extracellular domains of HLA class I (α1-α3, blue). The C-terminus consists of the constant domains 2 and 3 of the IgG heavy chain (CH2 and CH3, gray) or a fragment crystallizable (Fc). Disulfide bonds at the hinge region of Fc allow the homodimerization of HLA-Fc with bilateral symmetry. (B) The proposed mechanism of action for HLA-Fc. The HLA portion binds to cognate surface immunoglobulin (Ig, purple) and guides the fusion protein to corresponding antibody-producing cells; the Fc portion mediates cytotoxicity to enable target cell killing. (C) Components of HLA-Fc proteins used in the study. All variants start with a signal sequence, followed by a 9-mer peptide, flexible linker #1, human β2m (hβ2m), flexible linker #2, extracellular domains of the indicated HLA allele, and Fc of mouse IgG2a. The broken lines between linker #1 and the α2 domain represent disulfide bonds that secure the 9-mer peptides within the peptide-presenting domains (α1-α2). The A2Fc−LALAPG mutant contains 3 point mutations within the Fc portion (arrowheads). (D) Characterization of HLA-Fc proteins by polyacrylamide gel electrophoresis and Coomassie stain. HLA-Fc variants were purified with protein A from the supernatant of transiently transfected Expi293 cells and analyzed in nonreduced and reduced conditions.
We first constructed a prototype HLA-Fc comprising an A∗02:01 (A2) SCT and the Fc from mouse IgG2a. The A2 SCT consisted of an N-terminal signal peptide, a 9-mer peptide derived from cytomegalovirus phosphoprotein 65 (CMVpp65), a flexible linker, human β2-microglobulin, a second linker, and the extracellular domains of A2 (α1 through α3; Figure 1C; supplemental Figure 1). The signal peptide served as a cue to the secretory pathway and should be cleaved before secretion of the mature protein (supplemental Figure 2).27 This organization has previously been successful in the creation of SCTs for T-cell stimulation or detection purposes.28-31 The SCT in A2Fc also included a pair of cysteine residues to create a disulfide trap to secure the peptide in place22 (Figure 1C). As exemplified by A2Fc, the design of HLA-Fc was modular, with the potential of including diverse SCT and Fc components. To generate a negative control for the Fc component, we introduced 3 point mutations, collectively named LALAPG (Figure 1C), to disrupt Fc-mediated effector functions.24 We also constructed HLA-Fc with a different HLA specificity, B∗07:02 (B7) (Figure 1C), which is highly prevalent in humans and displays immunogenic epitopes distinct from A2.
We expressed the HLA-Fc variants in Expi293 cells and purified them from the supernatant by protein A chromatography at a yield of approximately 10 mg of protein per 1 L of culture. The size of nonreduced HLA-Fc was ∼180 kDa, as analyzed on a polyacrylamide gel; upon reduction by β-mercaptoethanol, the presumed monomers were detected as ∼90-kDa bands (Figure 1D). We also varied the 9-mer peptide sequences in B7Fc to determine the relationship between peptide affinity and HLA-Fc stability. Although the cleavage of signal peptide was unaffected by different 9-mer peptides (supplemental Figure 2; supplemental Table 2), only B7Fc variants carrying a high-affinity peptide could be stably expressed (supplemental Figure 3).
Blocking of anti-HLA antibodies in serum specimens by HLA-Fc
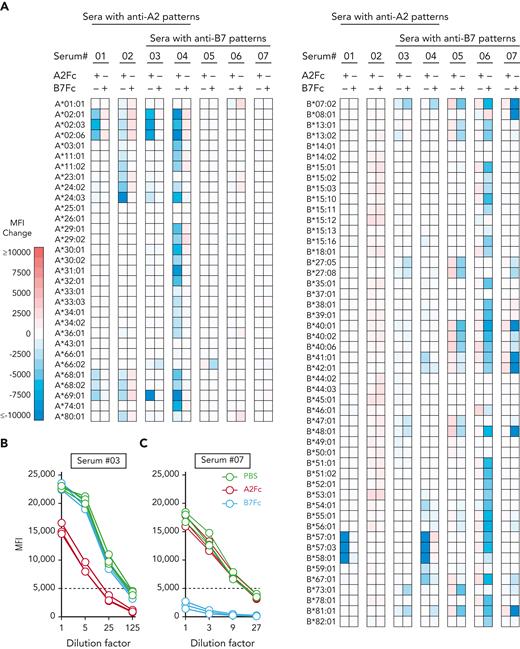
We hypothesized that if the structural features of the SCT in HLA-Fc were intact, A2Fc and B7Fc should be able to neutralize anti-A2 or -B7 antibodies in sera from alloimmunized transplant patients. We used a multiplexed SAB assay to determine the strength of anti-A2 and -B7 reactivities in patient sera (with or without the addition of HLA-Fc) as determined by the mean fluorescence intensity (MFI) of bead staining. In 4 sera with anti-A2 antibodies (#01-04), treatment with A2Fc, but not B7Fc, reduced the MFI values on A2-specific beads. In 5 sera with anti-B7 antibodies (#03-07), treatment with B7Fc, but not A2Fc, reduced the MFI values on B7-specific beads (Figure 2A; supplemental Figure 4).
Neutralization of cognate antibodies by HLA-Fc in clinical samples. (A) Changes in anti-A2 and -B7 reactivities in sera treated with A2Fc or B7Fc relative to PBS treatment. Clinical samples reactive to A2 (sera 01-02), or B7 (sera 05-07), or both (sera 03-04) were incubated with PBS, A2Fc, or B7Fc before testing by the single-antigen bead assay. The changes in mean fluorescence intensity (MFI) values after A2Fc or B7Fc treatment compared to PBS treatment are represented as a heatmap for each bead. Each row represents a bead specificity; the left and right panels show HLA-A- and HLA-B-specific beads, respectively. Red and blue hues indicate increased and decreased MFI, respectively. (B-C) Neutralization of anti-A2 and anti-B7 reactivities in titrated serum samples #03 (B) and #07 (C) by cognate HLA-Fc. Serial 1:5 dilutions were performed on the sera before incubation with PBS (green), A2Fc (red), or B7Fc (blue), followed by the single-antigen bead assay. The A2-specific reactivity in (B) was represented by MFI values from A∗02:01, A∗02:03, and A∗02:06 beads; The B7-specific reactivity in (C) was represented by B∗07:02, B∗42:01, and B∗81:01 beads (serum #07 is cross-reactive to these 3 antigens). Broken lines at 5000 MFI indicate the threshold associated with positive lymphocyte crossmatching.
Neutralization of cognate antibodies by HLA-Fc in clinical samples. (A) Changes in anti-A2 and -B7 reactivities in sera treated with A2Fc or B7Fc relative to PBS treatment. Clinical samples reactive to A2 (sera 01-02), or B7 (sera 05-07), or both (sera 03-04) were incubated with PBS, A2Fc, or B7Fc before testing by the single-antigen bead assay. The changes in mean fluorescence intensity (MFI) values after A2Fc or B7Fc treatment compared to PBS treatment are represented as a heatmap for each bead. Each row represents a bead specificity; the left and right panels show HLA-A- and HLA-B-specific beads, respectively. Red and blue hues indicate increased and decreased MFI, respectively. (B-C) Neutralization of anti-A2 and anti-B7 reactivities in titrated serum samples #03 (B) and #07 (C) by cognate HLA-Fc. Serial 1:5 dilutions were performed on the sera before incubation with PBS (green), A2Fc (red), or B7Fc (blue), followed by the single-antigen bead assay. The A2-specific reactivity in (B) was represented by MFI values from A∗02:01, A∗02:03, and A∗02:06 beads; The B7-specific reactivity in (C) was represented by B∗07:02, B∗42:01, and B∗81:01 beads (serum #07 is cross-reactive to these 3 antigens). Broken lines at 5000 MFI indicate the threshold associated with positive lymphocyte crossmatching.
Sera containing anti-A2 antibodies also bound to other cross-reactive beads in the multiplexed assay that shared epitopes with A2 (supplemental Figure 5). This provided an opportunity to evaluate multiple epitopes on A2Fc. A2Fc reduced the MFI on beads that cross-react with A2 beads, such as A∗69 (epitope 107W), A∗68 (epitope 142T/145H), and A∗11 (epitope 144K) among others (Figure 2A). The anti-B7 sera reacted with a set of epitopes distinct from the A2 epitopes (supplemental Figure 6), and, similarly, B7Fc reduced the MFI on those beads that cross-react with B7, such as B∗40 (epitope 177D/178K), B∗27 (epitope 163E/166E/167W), and B∗08 (epitopes 113H and 180E), among others (Figure 2A). These data support the presence of multiple intact epitopes on A2Fc and B7Fc proteins.
To evaluate the interaction between anti-HLA titers, MFI values, and the blocking effect of HLA-Fc, we performed serial 1:5 dilutions on representative sera (#03 and #07) followed by treatment with A2Fc or B7Fc (Figure 2B-C). We observed significant reductions in MFI at each titer after treatment by the cognate HLA-Fc but not the noncognate HLA-Fc. The anti-A2 antibody in serum #03 showed a saturating level of reactivity; only when diluted at 1:25 could A2Fc neutralize the anti-A2 reactivity to below the MFI threshold associated with a positive lymphocyte crossmatch.32 Although the above-mentioned results indicate the presence of functional epitopes on HLA-Fc proteins, they also highlight the complexity of clinical specimens with mixed polyclonal reactivities. To further test the therapeutic potential of HLA-Fc more specifically, we turned to B-cell hybridomas as a surrogate cellular target for HLA-Fc.
Binding of HLA-Fc to hybridoma cells of cognate specificities
PA2.1 and BB7.1 are hybridoma cells derived from mouse B lymphocytes with anti-A2 and -B7 specificities, respectively.33 The supernatant from PA2.1 cells primarily reacted with all 3 A∗02 beads and the A∗69:01 beads in the SAB assay (Figure 3A), indicating specificity for the 107W-associated epitope (supplemental Figure 5); there was also weak cross-reactivity with additional beads of HLA-A specificities but not HLA-B. The supernatant from BB7.1 cells primarily reacted with beads carrying B∗07:02, B∗42:01, and B∗81:01 (Figure 3A), indicating the involvement of 66I/69A/70Q- and/or 180E-associated epitopes (supplemental Figure 6). Moreover, the BB7.1 supernatant did not cross-react with A2-related antigens.
Selective binding of HLA-Fc to cognate hybridoma cells. (A) A2- and B7-specific reactivity of supernatants from PA2.1 and BB7.1 B cell hybridomas. Supernatants from the indicated cell culture media were tested by the single-antigen bead assay, with each row representing a bead specificity. Mean fluorescence intensity (MFI) values were graphed as a heatmap. HLA-A−specific and HLA-B−specific beads are shown in the left and right panels, respectively. (B) Binding of HLA-Fc to hybridoma cells. PA2.1 (upper panel of scatter plots) and BB7.1 (lower panel) cells were either untreated or treated with A2Fc, A2Fc-LALAPG, or B7Fc. Cells were stained with the indicated fluorochrome-conjugated antibodies (plots are pregated on viable cells [7-AAD−]). Numbers in scatter plots are the percentage of double-positive cells (IgG1+ and IgG2a+). All IgG subclasses were murine (mIgG). Plots are representative of at least 2 independent experiments.
Selective binding of HLA-Fc to cognate hybridoma cells. (A) A2- and B7-specific reactivity of supernatants from PA2.1 and BB7.1 B cell hybridomas. Supernatants from the indicated cell culture media were tested by the single-antigen bead assay, with each row representing a bead specificity. Mean fluorescence intensity (MFI) values were graphed as a heatmap. HLA-A−specific and HLA-B−specific beads are shown in the left and right panels, respectively. (B) Binding of HLA-Fc to hybridoma cells. PA2.1 (upper panel of scatter plots) and BB7.1 (lower panel) cells were either untreated or treated with A2Fc, A2Fc-LALAPG, or B7Fc. Cells were stained with the indicated fluorochrome-conjugated antibodies (plots are pregated on viable cells [7-AAD−]). Numbers in scatter plots are the percentage of double-positive cells (IgG1+ and IgG2a+). All IgG subclasses were murine (mIgG). Plots are representative of at least 2 independent experiments.
To test whether A2Fc and B7Fc selectively bound to hybridoma cells of cognate specificity, we incubated PA2.1 and BB7.1 cells with A2Fc, A2Fc-LALAPG, or B7Fc followed by flow-cytometric analysis (Figure 3B). Both cell lines produce IgG1 in both secreted and transmembrane forms, which could be distinguished from the IgG2a Fc domain of the HLA-Fc proteins. A2Fc and A2Fc-LALAPG bound exclusively to PA2.1 cells, with 95% of the cells being double positive. Conversely, B7Fc bound to 77.3% of BB7.1 cells but not PA2.1 cells.
Complement-dependent, antigen-specific killing of hybridoma cells by HLA-Fc
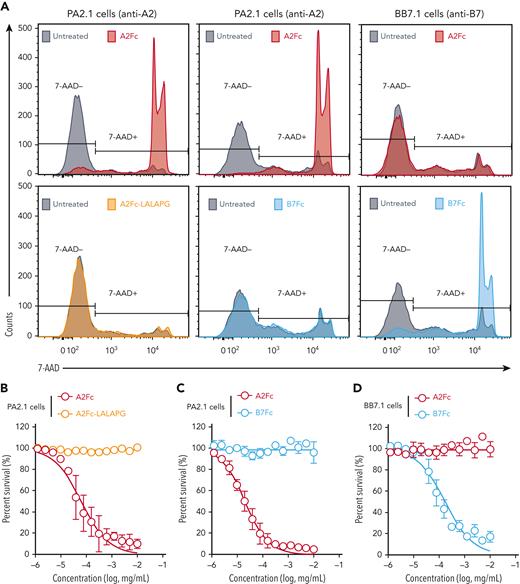
With the observed binding characteristics of HLA-Fc, we examined the feasibility of targeting cells with specific surface immunoglobulin for depletion while leaving bystander cells intact. We developed a CDC assay to evaluate the killing of antibody-producing hybridoma cells by HLA-Fc. Hybridoma cells were incubated with HLA-Fc in diluted rabbit serum as a source of complement factors for 3 hours at room temperature. Washed cells were subsequently stained with 7-AAD to quantify viable cells by flow cytometry. Although untreated PA2.1 cells were mostly viable, A2Fc treatment at 10−2 mg/mL killed most PA2.1 cells. In contrast, PA2.1 cells treated with A2Fc-LALAPG or B7Fc remained 7-AAD negative (Figure 4A, left and middle panels). The patterns reversed for BB7.1 cells, where B7Fc showed potent killing but A2Fc showed no effect (Figure 4A, right panels).
Complement-dependent, antigen-guided depletion of hybridoma cells by HLA-Fc in vitro. (A) Selective killing of PA2.1 cells and BB7.1 cells by cognate HLA-Fc. PA2.1 cells were untreated (gray) or treated with A2Fc (red), A2Fc-LALAPG (brown), or B7Fc (blue) at 0.01 mg/mL in diluted rabbit serum (left and middle panels); BB7.1 cells were untreated or treated with A2Fc or B7Fc at 0.01 mg/mL in diluted rabbit serum (right panels). After 3 hours of incubation at room temperature, washed cells were stained with 7-AAD and analyzed by flow cytometry to quantify viable cells. Overlaid histograms of 7-AAD staining are shown for untreated and treated cells as indicated; note that the upper left and upper middle panels represent the same condition from 2 independent experiments. (B-D) Dose relationship of HLA-Fc−mediated cytotoxicity on PA2.1 (B-C) and BB7.1 cells (D). Cells were treated with titrated HLA-Fc as indicated in diluted rabbit serum for 3 hours at room temperature, followed by 7-AAD staining and flow cytometry. The percentage of cell survival was normalized to that of untreated cells in each experiment. Data from 5 (B) and 3 (C-D) independent experiments. Circles and error bars represent mean ± SD. Curve fitting was performed by nonlinear regression using concentration vs normalized response and a variable slope model.
Complement-dependent, antigen-guided depletion of hybridoma cells by HLA-Fc in vitro. (A) Selective killing of PA2.1 cells and BB7.1 cells by cognate HLA-Fc. PA2.1 cells were untreated (gray) or treated with A2Fc (red), A2Fc-LALAPG (brown), or B7Fc (blue) at 0.01 mg/mL in diluted rabbit serum (left and middle panels); BB7.1 cells were untreated or treated with A2Fc or B7Fc at 0.01 mg/mL in diluted rabbit serum (right panels). After 3 hours of incubation at room temperature, washed cells were stained with 7-AAD and analyzed by flow cytometry to quantify viable cells. Overlaid histograms of 7-AAD staining are shown for untreated and treated cells as indicated; note that the upper left and upper middle panels represent the same condition from 2 independent experiments. (B-D) Dose relationship of HLA-Fc−mediated cytotoxicity on PA2.1 (B-C) and BB7.1 cells (D). Cells were treated with titrated HLA-Fc as indicated in diluted rabbit serum for 3 hours at room temperature, followed by 7-AAD staining and flow cytometry. The percentage of cell survival was normalized to that of untreated cells in each experiment. Data from 5 (B) and 3 (C-D) independent experiments. Circles and error bars represent mean ± SD. Curve fitting was performed by nonlinear regression using concentration vs normalized response and a variable slope model.
We measured the cytotoxicity of HLA-Fc over a range of concentrations, from 10−2 mg/mL to 1.22×10−6 mg/mL. For PA2.1 cells (Figure 4B-C), the half-maximal cytotoxic concentration (CC50) for A2Fc was 3.7 × 10−5 mg/mL (95% CI, 3.0-4.6 × 10−5 mg/mL), whereas A2Fc-LALAPG and B7Fc exhibited no significant cytotoxicity at the highest concentration tested. For BB7.1 cells (Figure 4D), the CC50 for B7Fc was 1.7 × 10−4 mg/mL (95% CI, 1.3-2.4 × 10−4 mg/mL), whereas A2Fc did not exhibit significant cytotoxicity at the highest concentration tested. To examine the effect of A2Fc on a mixed cell population, we added A2Fc or A2Fc-LALAPG to combined PA2.1 or BB7.1 cells with either cell line labeled with carboxyfluorescein succinimidyl ester (CFSE). A2Fc selectively killed PA2.1 cells but not BB7.1 cells regardless of which cell line was CFSE labeled, whereas A2Fc-LALAPG showed no cytotoxicity (supplemental Figure 7A-B); moreover, rabbit serum was necessary as a complement source for the effect of A2Fc (supplemental Figure 7C). These results established the antigen- and Fc-dependent killing of antibody-producing cells by cognate HLA-Fc in vitro.
Depletion of PA2.1 cells by A2Fc in vivo
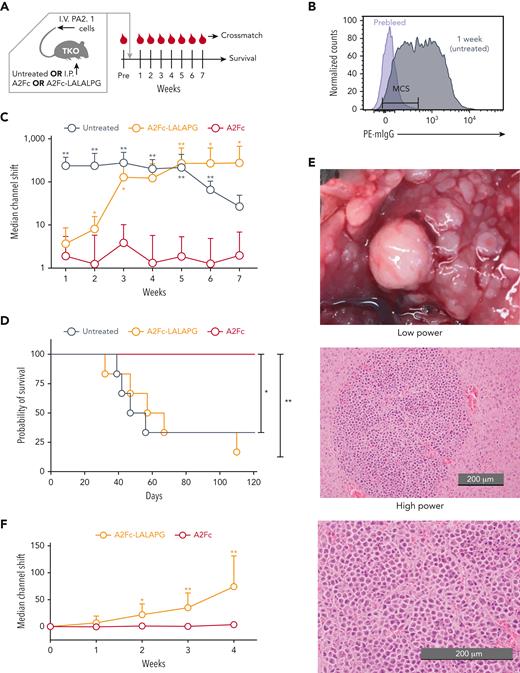
To examine the function of HLA-Fc in vivo, we transferred PA2.1 cells via tail vein injection into TKO mice, a strain of immunodeficient mice that lack T and B cells due to RAG2 deficiency and natural killer (NK) cells due to IL2RG deficiency, and allow improved engraftment of transplanted cells in some settings due to CD47 deficiency. Transferred hybridoma cells were able to engraft in these mice and to produce circulating anti-A2 antibodies. By systemically administering A2Fc or A2Fc-LALAPG via intraperitoneal injection, we were able to assess whether A2Fc can overcome circulating anti-A2 and deplete target cells by fixing endogenous complement factors (Figure 5A). We selected a dose of HLA-Fc protein (0.4 mg, equivalent to 0.016 g/kg in a 25-g mouse) that is comparable to the dose of rituximab commonly used in humans (1 g/70 kg body weight, or ∼0.014 g/kg). Each cohort of mice was bled before the cell transfer (prebleed) and weekly for 7 weeks. Sera were separated and examined by flow-cytometric crossmatching against splenocytes from A2-transgenic mice. The circulating anti-A2 levels were quantified as the shift of median fluorescence intensity (or MCS) relative to the prebleed specimens as the baseline control (Figure 5B). In untreated TKO mice, we observed an abrupt and sustained increase in circulating anti-A2 levels beginning the first week after the transfer of PA2.1 cells (Figure 5C). With A2Fc-LALAPG treatment, the anti-A2 levels were dampened in the first 2 weeks, compared with untreated mice, but increased by approximately 10-fold by the third week and remained at high levels in the subsequent weeks. These kinetics imply an initial blockade of anti-A2 by A2Fc-LALAPG, which was overwhelmed by the subsequent outgrowth of PA2.1 cells. With A2Fc treatment, the anti-A2 levels were ∼2-log lower than those of the untreated mice throughout the experiment, suggesting successful depletion of PA2.1 cells in vivo. In addition to the flow-cytometric crossmatch, we also verified the A2-specific reactivity of sera from untreated and A2Fc-LALAPG−treated representative mice, and the elimination of this reactivity by A2Fc treatment, using the SAB assay (supplemental Figure 8).
Depletion of A2-specific hybridoma cells by A2Fc in vivo. (A) TKO mice were injected with 1 million PA2.1 cells per mouse intravenously followed by no treatment or A2Fc or A2Fc-LALAPG via intraperitoneal injection at 0.4 mg per mouse. The untreated group did not receive any placebo. Mice were bled before cell transfer and treatment (Pre) and then weekly thereafter. Mice were followed for survival, and serially collected serum specimens were tested by crossmatching with splenocytes from A2 transgenic mice. (B) Representative crossmatching on A2 transgenic splenocytes. Cells were stained with serum from a mouse prior to injection of PA2.1 cells (prebleed; purple) or serum from a mouse that carried PA2.1 cells for 1 week but received no HLA-Fc treatment (gray), followed by staining with phycoerythrin (PE)−conjugated anti-mouse IgG (mIgG). Gating on CD3+ T cells allowed detection of anti-A2 antibody, which could be quantified by the median channel shift (MCS) of fluorescent intensity relative to the prebleed specimen. (C) Levels of circulating anti-A2 antibody monitored over time in TKO recipients of PA2.1 cells that were untreated or treated with A2Fc or A2Fc-LALAPG, as measured by crossmatching against A2+ splenocytes, represented as the median channel shift. Data are from 5 to 6 mice per group from 3 experiments. Mean ± SD. (D) Survival of mice following PA2.1 cell infusion and treatment with A2Fc or A2Fc-LALAPG or no treatment. Data are from 6 mice per group from 3 experiments. (E) Multifocal tumors formed by hybridoma cells in the liver of representative untreated and A2Fc-LALAPG−treated mice (gross examination, upper panel; microscopic examination of hematoxylin and eosin−stained sections, middle and lower panels). (F) Immunogenicity of A2Fc and A2Fc-LALAPG. B6 mice were treated with the same dose of A2Fc or A2Fc-LALAPG via intraperitoneal injection as in (C), and anti-A2 levels in sera collected before and weekly after the treatment were measured by crossmatching against A2+ splenocytes. Median channel shifts are from 6 mice per group from 2 experiments. Mean ± SD; SDs for the A2Fc group were smaller than the symbols in the plot. ∗P < .05; ∗∗P < .01, compared with the A2Fc-treated group by Mann-Whitney test (C,F) and log-rank test (D).
Depletion of A2-specific hybridoma cells by A2Fc in vivo. (A) TKO mice were injected with 1 million PA2.1 cells per mouse intravenously followed by no treatment or A2Fc or A2Fc-LALAPG via intraperitoneal injection at 0.4 mg per mouse. The untreated group did not receive any placebo. Mice were bled before cell transfer and treatment (Pre) and then weekly thereafter. Mice were followed for survival, and serially collected serum specimens were tested by crossmatching with splenocytes from A2 transgenic mice. (B) Representative crossmatching on A2 transgenic splenocytes. Cells were stained with serum from a mouse prior to injection of PA2.1 cells (prebleed; purple) or serum from a mouse that carried PA2.1 cells for 1 week but received no HLA-Fc treatment (gray), followed by staining with phycoerythrin (PE)−conjugated anti-mouse IgG (mIgG). Gating on CD3+ T cells allowed detection of anti-A2 antibody, which could be quantified by the median channel shift (MCS) of fluorescent intensity relative to the prebleed specimen. (C) Levels of circulating anti-A2 antibody monitored over time in TKO recipients of PA2.1 cells that were untreated or treated with A2Fc or A2Fc-LALAPG, as measured by crossmatching against A2+ splenocytes, represented as the median channel shift. Data are from 5 to 6 mice per group from 3 experiments. Mean ± SD. (D) Survival of mice following PA2.1 cell infusion and treatment with A2Fc or A2Fc-LALAPG or no treatment. Data are from 6 mice per group from 3 experiments. (E) Multifocal tumors formed by hybridoma cells in the liver of representative untreated and A2Fc-LALAPG−treated mice (gross examination, upper panel; microscopic examination of hematoxylin and eosin−stained sections, middle and lower panels). (F) Immunogenicity of A2Fc and A2Fc-LALAPG. B6 mice were treated with the same dose of A2Fc or A2Fc-LALAPG via intraperitoneal injection as in (C), and anti-A2 levels in sera collected before and weekly after the treatment were measured by crossmatching against A2+ splenocytes. Median channel shifts are from 6 mice per group from 2 experiments. Mean ± SD; SDs for the A2Fc group were smaller than the symbols in the plot. ∗P < .05; ∗∗P < .01, compared with the A2Fc-treated group by Mann-Whitney test (C,F) and log-rank test (D).
TKO mice in the untreated and A2Fc-LALAPG-treated groups began to succumb to outgrowth of PA2.1 cells at 4 weeks after cell transfer (Figure 5D). Gross examination of morbid mice revealed multifocal hepatic and splenic masses (Figure 5E, left). On histologic examination, we observed sheets of hybridoma cells with high nucleus-cytoplasm ratios and frequent mitotic figures (Figure 5E, middle and right). In contrast, all A2Fc-treated mice survived beyond 120 days (Figure 5D), and no gross masses were found on necropsy.
We also tested the immunogenicity of A2Fc and A2Fc-LALAPG in immunocompetent B6 mice. When tested at the same dose and route as used in TKO mice, A2Fc did not stimulate production of anti-A2 as measured by flow-cytometric crossmatch against A2+ splenocytes. Interestingly, A2Fc-LALAPG stimulated modest production of anti-A2 immunization (Figure 5F), indicating that a functional Fc may have inhibited alloimmunization to A2Fc.
Mitigation of anti-A2−mediated platelet transfusion refractoriness by A2Fc
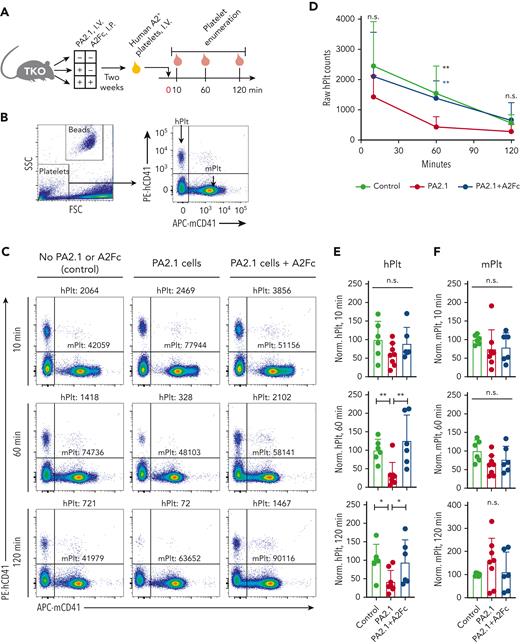
Because A2Fc treatment lowered anti-A2 levels in TKO mice carrying PA2.1 cells, we hypothesized that A2Fc could mitigate anti-A2-mediated refractoriness to A2-expressing (A2+) human platelets. We transfused A2+ platelets into TKO mice 10 to 14 days after PA2.1 cell transfer, with or without A2Fc administered at the time of cell transfer. Recipient mice were bled at 10, 60, and 120 minutes following platelet transfusion to enumerate circulating platelets (Figure 6A). Host and donor platelets were distinguished by staining for murine and human glycoprotein IIb (CD41), respectively (Figure 6B). We observed diminished counts of human platelets in PA2.1-carrying TKO mice at 60 and 120 minutes compared to control mice (not carrying PA2.1 cells), whereas human platelet increments in A2Fc-treated mice were comparable to those in control mice (Figure 6C). We quantified both raw platelet counts and normalized counts across multiple experiments. Transfused human A2+ platelets steadily decayed at similar rates in control and A2Fc-treated mice, whereas platelets abruptly decreased at 60 minutes in untreated, PA2.1-carrying mice (Figure 6D). When platelet counts were normalized to counts in control mice, human platelet counts were diminished in untreated, PA2.1-carrying mice at both 60 and 120 minutes (Figure 6E). Normalized murine platelet counts showed no significant decrease among the groups of mice at any time point (Figure 6F).
Attenuation of A2-specific platelet transfusion refractoriness by A2Fc. (A) TKO mice were divided into 3 groups with different combinations of PA2.1 cell infusion and A2Fc treatment, as indicated. The minus signs (−) mean no cell infusion or A2Fc treatment; no placebo was administered. Two weeks after cell infusion and treatment, A2+ human platelets were transfused via intravenous injection, followed by bleeding at 10, 60, and 120 minutes for platelet enumeration. (B) Enumeration of human and murine platelets (hPlt and mPlt) by flow cytometry. Representative gating for platelets and CountBright absolute counting beads on the left. Right panel: hPlt and mPlt were distinguished by staining for hCD41 and mCD41 markers, respectively. (C) Representative flow cytometry of platelets in the indicated mice. Posttransfusion counts of hPlt and mPlt at 10, 60, and 120 minutes are shown (number of platelets per 5000 counting beads). (D) Time course of raw hPlt counts for 3 groups of TKO mice. Mean ± SD. (E-F) Normalized hPlt counts (E) and mPlt counts (F) at 10, 60, and 120 minutes for the indicated groups of mice. Counts were normalized to mean platelet counts from control mice as 100% at each time point within each experimental cohort. Points are normalized counts; bars and error bars are mean ± SD. (D-F) Data are from 6 to 8 mice per group from 3 experiments. n.s., not significant; ∗P < .05; ∗∗P < .01, compared with PA2.1 by Mann-Whitney test.
Attenuation of A2-specific platelet transfusion refractoriness by A2Fc. (A) TKO mice were divided into 3 groups with different combinations of PA2.1 cell infusion and A2Fc treatment, as indicated. The minus signs (−) mean no cell infusion or A2Fc treatment; no placebo was administered. Two weeks after cell infusion and treatment, A2+ human platelets were transfused via intravenous injection, followed by bleeding at 10, 60, and 120 minutes for platelet enumeration. (B) Enumeration of human and murine platelets (hPlt and mPlt) by flow cytometry. Representative gating for platelets and CountBright absolute counting beads on the left. Right panel: hPlt and mPlt were distinguished by staining for hCD41 and mCD41 markers, respectively. (C) Representative flow cytometry of platelets in the indicated mice. Posttransfusion counts of hPlt and mPlt at 10, 60, and 120 minutes are shown (number of platelets per 5000 counting beads). (D) Time course of raw hPlt counts for 3 groups of TKO mice. Mean ± SD. (E-F) Normalized hPlt counts (E) and mPlt counts (F) at 10, 60, and 120 minutes for the indicated groups of mice. Counts were normalized to mean platelet counts from control mice as 100% at each time point within each experimental cohort. Points are normalized counts; bars and error bars are mean ± SD. (D-F) Data are from 6 to 8 mice per group from 3 experiments. n.s., not significant; ∗P < .05; ∗∗P < .01, compared with PA2.1 by Mann-Whitney test.
Discussion
Donor-specific anti-HLA antibodies are major causes of platelet transfusion refractoriness, primary graft failure after HLA-mismatched HSCT, and rejection following solid organ transplantation.16,34,35 Anti-HLA antibody−mediated platelet refractoriness demands recruitment of compatible or HLA-matched donors,36 which is difficult for highly sensitized patients.35 Anecdotal uses of plasmapheresis, IV immunoglobulin, rituximab, or bortezomib have been reported for platelet refractoriness37-39 and warrant further studies. In mismatched HSCT, DSA increases the risk of primary graft failure by 7-fold, as summarized in a recent meta-analysis.40 Several single-center observational studies showed mixed but encouraging results with various desensitization regimens before HSCT, incorporating plasmapheresis, IV immunoglobulin, rituximab, bortezomib, tacrolimus, and mycophenolate mofetil.41-46 However, clinical trials evaluating the efficacy of some of these treatments for DSA in solid organ transplant recipients, such as plasmapheresis, rituximab and bortezumib, have not confirmed their benefits.47 Importantly, none of these conventional therapies can target HLA-specific antibody-producing cells without compromising global humoral immunity.
To address the unmet need of a therapy that specifically targets anti-HLA antibody-mediated disorders, we created and characterized a prototype recombinant protein consisting of class I HLA and Fc from IgG. HLA-Fc represent a new class of modular proteins that can be rationally designed, with the potential of expanding into a panel of biologics to cover additional antigen specificities and Fc variants. As homodimers carrying bivalent antigens in the form of HLA single-chain trimers, HLA-Fc proteins can reduce antigen-specific serological reactivities in clinical samples and selectively bind to B-cell hybridomas of cognate specificities. HLA-Fc proteins mediate antigen-specific and Fc-dependent cytotoxicity on target cells in vitro, with minimal killing of nontarget cells. In immunodeficient mice seeded with hybridoma cells, HLA-Fc can deplete target cells in vivo, prevent their outgrowth, and lower the level of circulating antibodies secreted by these cells. By reducing anti-HLA antibodies in vivo, HLA-Fc can also improve the response to platelets bearing the corresponding HLA in a human-to-murine platelet transfusion model. Thus, HLA-Fc may provide a novel, off-the-shelf solution to the root cause of DSA-related conditions without global humoral suppression.
One of the critical molecular events of HLA-Fc−based therapy is the interaction between the HLA portion of the recombinant protein with cognate surface Ig or circulating antibody. We have detailed our strategy for designing the antigen portion of HLA-Fc in this study; notably, a 9-mer peptide with a high affinity for the linked HLA molecular appeared to be necessary for stable expression of a particular HLA-Fc. Although an HLA molecule may present many 9-mer peptides, we suspect that the impact of peptide sequences on the efficacy of HLA-Fc is probably small. Although peptide-specific anti-HLA antibodies have been reported in B-cell clones isolated from patient samples,48 state-of-the-art clinical assays do not detect peptide-specific antibodies without losing predictive value.2,49 Current epitope analysis approaches also focus on solvent-accessible polymorphisms on the surface of HLA molecules.50-53
The other crucial molecular event for HLA-Fc action is the protein’s Fc-mediated effector function. Our study focused on characterizing the potential of HLA-Fc to trigger CDC in vitro using rabbit serum as a complement source, and this effect was abolished with an Fc variant incapable of fixing complement.24 We conclude that CDC is sufficient for HLA-Fc−mediated killing of target cells, which in this sense is similar to the primary mechanism for rituximab-mediated depletion of CD20+ B cells.54 Interestingly, even when paired with an Fc variant lacking complement-dependent or Fc-γ−dependent effector function, the antigen portion of HLA-Fc may temporarily lower the level of cognate antibodies in vivo (Figure 5C) and shield grafts from DSA-mediated attack. Our in vivo results suggest that HLA-Fc can induce Fc-dependent cytotoxicity using endogenous murine complement factors, given that our experiments in TKO mice provide a system lacking all host lymphocytes, including NK cells. Nevertheless, other complement-independent effector functions, such as Fc receptor−mediated cellular cytotoxicity and phagocytosis, may also contribute to the effectiveness of HLA-Fc in immunocompetent hosts.
This proof-of-concept study has a few limitations. First, we used B-cell hybridomas as a model to test the effect of HLA-Fc on cognate antibody-producing cells in vitro and in vivo. Future studies could use appropriate alloimmunization models, ideally in mice carrying HLA transgenes and producing anti-HLA antibodies with patterns and strengths similar to those seen in transplant patients. These more physiological models will enable the evaluation of HLA-Fc’s effect on antigen-specific B cells. Second, HLA-Fc may be neutralized by high levels of circulating anti-HLA antibodies (Figure 2B), which would thwart the intended targeting of cognate B cells. However, adding plasmapheresis, IgG-degrading agents (eg, imlifidase), and T-cell suppression as part of a multi-modality regimen may increase the opportunity for HLA-Fc to reach target cells; administrating higher doses of HLA-Fc may also be necessary. Third, our approach may not remove DSA-producing plasma cells because of their lack of surface Ig. It would be important to understand the lifespan of these plasma cells in a relevant alloimmunization model, as this could impact the efficacy of HLA-Fc therapy. Nevertheless, we reason that targeting donor-specific memory B cells would deplete the precursors of donor-specific plasma cells and, therefore, benefit patients in the long term. One technical limitation of our study was that we could not quantify the concentrations of anti-HLA antibodies. Both the SAB and flow crossmatch assays are semi-quantitative, and MFI and MCS values should be interpreted as such. Finally, we have not explored the development of class II HLA-Fc to treat conditions mediated by class II DSA.
In summary, we have shown that HLA-Fc selectively deplete antigen-specific antibody-producing cells via Fc-dependent effector function. Our findings serve as the prelude to a new line of investigation to improve and diversify HLA-Fc proteins to overcome unwanted alloantibodies encountered in allogeneic transfusion and transplantation. Because HLA-Fc differs from traditional lineage-based cell elimination, this approach may help redefine precision medicine in histocompatibility to enable more flexible use of allogeneic cells and organs with improved outcomes.
Acknowledgments
The authors thank Paul Allen, Ali Ellebedy, Thaddeus Stappenbeck, and Charles Eby for their scientific advice; Melissa Cook, Rick Stegeman, and Brian Duffy for their technical assistance; and volunteers for donating blood for platelet transfusions.
This work was supported by the Foundation for Barnes-Jewish Hospital (Award Reference Number: 4082; C.L.), a Mallinckrodt-Washington University Challenge Grant from the Edward Mallinckrodt Jr Foundation (C.L.), and the National Institutes of Health, National Institute of Allergy and Infectious Diseases (AI168706; B.T.E. and C.L.).
Authorship
Contribution: D.H.F., B.T.E., and C.L. conceived and designed the study; A.M.W., T.R.B., H.G., C.A.N., and C.L. acquired the data; A.M.W., X.W., D.H.F., B.T.E., and C.L. analyzed and interpreted the data; A.M.W., B.T.E., and C.L. drafted the manuscript; A.M.W., T.R.B., X.W., H.G., C.A.N., D.H.F., B.T.E., and C.L. critically revised and edited the manuscript; and X.W. and D.H.F. provided key materials.
Conflict-of-interest disclosure: C.L., D.H.F., B.T.E, X.W., and C.A.N. are coinventors on a patent application related to HLA-Fc filed by Washington University in St Louis. The remaining authors declare no competing financial interests.
Correspondence: Brian T. Edelson, Department of Pathology and Immunology, Washington University School of Medicine, 425 South Euclid Ave, MSC 8118-0004-08, St Louis, MO 63110; e-mail: bedelson@wustl.edu; and Chang Liu, Department of Pathology and Immunology, Washington University School of Medicine, 425 South Euclid Ave, MSC 8118-0004-05, St Louis, MO 63110; e-mail: cliu32@wustl.edu.
References
Author notes
Data are available on request from the corresponding authors.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by pagecharge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.


![Selective binding of HLA-Fc to cognate hybridoma cells. (A) A2- and B7-specific reactivity of supernatants from PA2.1 and BB7.1 B cell hybridomas. Supernatants from the indicated cell culture media were tested by the single-antigen bead assay, with each row representing a bead specificity. Mean fluorescence intensity (MFI) values were graphed as a heatmap. HLA-A−specific and HLA-B−specific beads are shown in the left and right panels, respectively. (B) Binding of HLA-Fc to hybridoma cells. PA2.1 (upper panel of scatter plots) and BB7.1 (lower panel) cells were either untreated or treated with A2Fc, A2Fc-LALAPG, or B7Fc. Cells were stained with the indicated fluorochrome-conjugated antibodies (plots are pregated on viable cells [7-AAD−]). Numbers in scatter plots are the percentage of double-positive cells (IgG1+ and IgG2a+). All IgG subclasses were murine (mIgG). Plots are representative of at least 2 independent experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/140/16/10.1182_blood.2022016376/13/m_blood_bld-2022-016376-gr3.jpeg?Expires=1765950557&Signature=JQ7MdFUul3HhdOqx0bTFnH6gwD6OXRLzz2B8mhfuVNP5tJZMpEUolF~oJ7Ww6VK4KfyPu1FgDbSiYgqRrI-2yEku0YASplUjQKCJZLaa13YJtIBSgWKwg4lThZEqTO3ROiy9UciZQxxZuXA5HN5LNUuIdlzhNYhLgLDWGjqkNoZ7LHjQQwhUh2AihCPB7uT59o1G~BQDp3nYr-syEPYdJLZB0PIQdMS0Z~sC-tjoCWxnj9EzKS2KkevhPMXhvEuxzjV0i97vTBGyhdstKzTDUEvppZ3ZK4QbeVifdUro~4uGKYoopCdxFeBso8EUpA-Zhv72vVP5hPhdT7L3rHUZrw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)