In this issue of Blood, Maciocia et al1 report the identification of C-C motif chemokine receptor 9 (CCR9), a chemokine receptor expressed on a small subset of normal T cells, as a promising target for T-cell acute lymphoblastic leukemia (T-ALL)–directed chimeric antigen receptor (CAR) T-cell therapy.
Aside from protecting us from external pathogenic threats, our immune systems have the power to recognize and destroy premalignant and malignant cells based on recognition of missing self and transformation-induced expression of cancer neoantigens. Evasion of a productive tumor immune response is recognized as a hallmark of cancer2 and is required for overt malignant transformation. Immunotherapies such as checkpoint blockade molecules and bispecific antibodies have revolutionized the treatment of cancer by reactivating tumor immunosurveillance to fight malignancy. Furthermore, decades of biological research into T-cell receptor signaling has allowed researchers to engineer tumor antigen-specific CARs—an antibody and T-cell receptor hybrid—that when expressed on patient-derived T cells results in a potent and sustained antitumor response.3
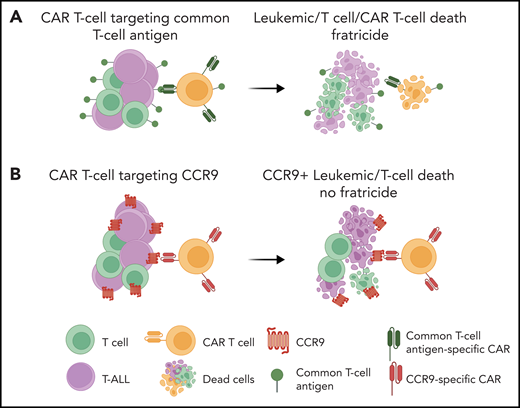
The poster child for immunotherapeutic success is the development of CAR T cells such as those targeting B-cell–specific antigen CD193 for treating relapsed or refractory B-cell acute lymphoblastic leukemia (B-ALL). The backbone of the success of these common B-antigen targeted therapies for the treatment of B-ALL is our ability to tolerate B-cell aplasia. Unlike B-cell aplasia, T-cell aplasia displays intolerable immunosuppression, which precludes the adoption of immunotherapies that target T-cell antigens for treating T-ALL. Thus, a major hurdle for the adoption of CAR T-cell therapy for the treatment of T-ALL is the inability to find T-ALL antigens that are not expressed on nonmalignant T cells. Not only is T-cell aplasia detrimental to survival, CAR T cells engineered to recognize T-cell antigens will inevitably turn on themselves, resulting in CAR T-cell lysis, termed “fratricide,”4 from the Latin term for the killing of one’s brother (see figure panel A). To circumvent fratricide, Maciocia et al first embarked on a hunt for potential cell surface antigens unique to T-ALL cells (see figure panel B).
Anti-CCR9 CAR T cells for T-ALL. (A) A major challenge for the adoption of CAR T-cell therapy for T-ALL is the inability to find T-ALL–specific antigens that are not expressed on normal T cells. Targeting common T-cell antigens would lead to T-cell fratricide and a profound immunosuppression. (B) Maciocia et al report the identification of CCR9, a chemokine receptor expressed on a small subset of normal T cells, as a promising target for T-ALL–directed CAR T-cell therapy. Schematic was created with Biorender.
Anti-CCR9 CAR T cells for T-ALL. (A) A major challenge for the adoption of CAR T-cell therapy for T-ALL is the inability to find T-ALL–specific antigens that are not expressed on normal T cells. Targeting common T-cell antigens would lead to T-cell fratricide and a profound immunosuppression. (B) Maciocia et al report the identification of CCR9, a chemokine receptor expressed on a small subset of normal T cells, as a promising target for T-ALL–directed CAR T-cell therapy. Schematic was created with Biorender.
The authors reasoned that comparing gene expression profiles of 35 normal tissues and the MOLT-4 T-ALL cell line included in the Human Protein Atlas cancer compendium would allow a subtractive transcriptomics approach for identifying genes uniquely expressed in T-ALL cells. By using this approach, the team identified CCR9, a cell surface chemokine receptor, as a potential target for CAR T-cell therapy. Next, flow cytometry analysis demonstrated that the majority of pediatric patients (72%) and adult patients (75%) with primary T-ALL express CCR9. Finally, to address the elephant in the room, the authors demonstrated that CCR9 is expressed on only 11% of B cells and 5% of T cells from human peripheral blood. In addition, protein or transcript is undetected on other mature cell subsets in blood or in CD34+ marrow cells and thymic subsets, respectively. Thus, given the limited numbers of nonmalignant cells expressing CCR9, the authors predicted that targeting CCR9 would mitigate CAR T-cell fratricide.
CCR9, the receptor for C-C motif chemokine ligand 25 (CCL25), is expressed on a minority of mature T cells, mostly localized to the gut, and on some immature thymocytes in mice and humans.5 CCL25 is mainly expressed in the thymus and gut, where its expression regulates the homing and retention of thymocytes, and a small subset of B and T cells in these tissues, respectively. Interestingly, a previous study found that NOTCH1 signaling, which is activated by mutation in ∼70% of patients with T-ALL,6 regulates CCR9 expression in T-ALL cell lines and primary samples,7 supporting the authors’ discovery of its potential as an immunotherapeutic target in T-ALL.
Next, the authors developed a CCR9-specific antibody by gene-gun vaccination of rats with a plasmid encoding human CCR9. After identifying a CCR9-specific hybridoma, the authors generated a single-chain variable fragment for downstream cloning as a second-generation CAR, which they designate CARCCR9. Expression of this CAR on primary human T cells did not result in fratricide. Further in vitro characterization of the CARCCR9 T-cell function demonstrated CARCCR9 T-cell cytotoxic activity against CCR9+ but not CCR9– T-ALL cell lines and primary T-ALL cells.
To demonstrate the in vivo tumor clearance potential of CARCCR9 cells, the authors next used cell-line and patient-derived xenograft (PDX) mouse models of T-ALL. Immunodeficient NOD scid gamma (NSG) mice were engrafted by intravenous injection of firefly-luciferase expressing MOLT-4 T-ALL cells, and engraftment progress was tracked by bioluminescence imaging. As expected, mice treated with CARCCR9 T cells, but not untreated mice or mice treated with anti-CD19 CAR T cells, exhibited extended survival. Furthermore, the authors demonstrated the persistence of CARCCR9 by re-challenging mice with MOLT-4 cells 40 days after initial treatment with CARCCR9 and found that 75% of mice remained negative for leukemia. Finally, the authors repeated their CARCCR9 treatment study by generating 6 different PDX mouse models from primary T-ALL samples with various levels of CCR9 expression. Once again, treatment with CARCCR9 significantly extended mouse survival, and minimal graft-versus-host disease was observed, which highlights the preclinical efficacy of the CARCCR9 therapy developed by the authors.
Overall, by starting with a subtractive transcriptomics approach, the authors have added a new target to the anticancer CAR T-cell arsenal. Aside from the promise of this new CAR T-cell therapy for treating T-ALL, the subtractive transcriptomics approach used by the authors has the potential to be applied successfully across a wide range of tumors. The next stages of development of this novel therapy will hold new challenges. Although the human and mouse T cells profiled in this study do not primarily express CCR9, immunophenotypes of mouse and human T cells are known to be different, and the immune landscape in human tissues (aside from peripheral blood) might better represent these differences.8 Although eliminating tissue-resident and innate T-cell populations should not result in the same dismal clinical outcomes as activating them,9 it remains to be seen whether CCR9+ T cells play a greater role in human tissue homeostasis than in mouse tissue. Ultimately, all translational research that adds to our understanding of antitumor immunity will offer an invaluable chance to further characterize human immune cell populations.
Conflict-of-interest disclosure: The authors declare no competing financial interests.