In this issue of Blood, DeAngelo et al1 report the promising results of targeting the bone marrow vascular niche with the endothelial selectin (E-selectin) inhibitor uproleselan in combination with intensive chemotherapy in a phase 1/2 single-arm trial in acute myeloid leukemia (AML). They noted no additive toxicity and encouraging response rates in both the relapsed/refractory (R/R) and first-line settings.
Resistance to cytotoxic therapy and the persistence of leukemia stem cells (LSCs) that drive relapse have been formidable challenges in the field of AML. Non-immunologic leukemia-microenvironmental interactions have been demonstrated to support leukemia cells escape from cytotoxic chemotherapy. Several bone marrow stromal interactions may be amenable to therapeutic modulation including adhesion molecule-ligand interactions such as VLA4-VCAM-1/fibronectin, E-selectin/sialylated carbohydrates, the CXCR4/CXCL12 signaling axis, among others.2 Among these, CXCR4 inhibitors have been extensively studied in the clinic in combination with cytotoxic chemotherapy for AML with modest success. Such combinations have shown composite complete remission (CRc) rates of 39% to 46% and median overall survival (OS) of 8.2 to 10.8 months in R/R AML.3 There are no CXCR4 inhibitors which are currently in advanced clinical development for AML.
E-selectin (CD62E) is a vascular adhesion molecule expressed on the vascular endothelium that helps leukocytes stick to the vessel walls and facilitates leukocyte trafficking. In addition, it is involved in the pathophysiology of venous thrombosis, acute coronary syndrome, cerebral aneurysm, and pathogen attachment. LSCs bind to endothelial E-selectin in the marrow microenvironment through ligands, including CD44, CD162, and L-selectin, and this pathway may also be involved in self-sustaining paracrine signaling.4 Such interactions contribute to the sheltering of LSCs in the marrow vascular niche, promoting LSC quiescence, escape, and eventual resistance to cytotoxic chemotherapy. These effects may be abrogated by disrupting E-selectin interactions.5
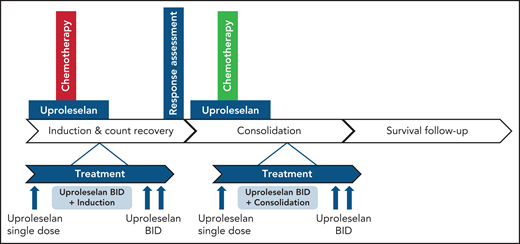
The authors leveraged these insights to combine E-selectin inhibitor uproleselan with intensive chemotherapy that included mitoxantrone, etoposide, and cytarabine (MEC) in the salvage setting in patients age 18 years or older and cytarabine and idarubicin (7 + 3) in the first-line setting in patients age 60 years or older in a phase 1/2 single-arm trial (see figure). Uproleselan showed no dose-limiting or additive toxicities when administered with chemotherapy. Rates of adverse events were as expected with MEC and 7 + 3 alone. Sixty-day mortality in R/R AML was 9%, and in newly diagnosed AML, it was 12%. The median time to neutrophil recovery to 0.5 ×109/L and platelet recovery to 50 ×109/L were 32 to 33 days, respectively. Interestingly, rates of mucositis were lower than expected with intensive chemotherapy, potentially because inflammatory responses were blocked by inhibition of E-selectin. The CRc rate was 41% (n = 21 of 54), the CR rate was 35% (19 of 54), and median OS in R/R AML was 8.8 months. In newly diagnosed AML, CRc was 81% (n = 18 of 25), CR was 52% (n = 13 of 25), and median OS was 12.6 months. The CRc rates in patients with R/R AML with a remission duration of <12 vs ≥12 months were 28% and 83%, respectively, suggesting a preferential activity in patients with late relapse. An exploratory analysis suggested that outcomes may be better in patients with pretreatment AML blasts that have >10% expression of E-selectin ligands suggesting the potential utility of E-selectin as a biomarker.
Treatment schema of uproleselan in combination with intensive chemotherapy for AML. See Figure 1 in the article by DeAngelo et al that begins on page 1135.
Treatment schema of uproleselan in combination with intensive chemotherapy for AML. See Figure 1 in the article by DeAngelo et al that begins on page 1135.
Of note, the R/R AML cohort was a relatively young population with a median age of 59 years (range, 26-84 years) with 67% having received only 1 previous line of therapy (salvage 1). The newly diagnosed patients had a median age of 67 years (range, 60-79 years) with 76% having unfavorable risk AML according to European LeukemiaNet. Event-free survival (EFS) in the R/R AML population was relatively short at 1.5 months with a modest median OS of 5.9 months in patients with early relapse (<12 months) compared with median OS not reached in patients with late relapse (>12 months).
Caution is necessary for making cross-trial comparisons, but these response rates and median OS are encouraging when compared with historical expectations. In patients younger than age 60 years who have R/R AML, historical outcomes with intensive chemotherapy include CRc rates of 10% to 40% and median OS of 3 to 8 months.6 In patients with newly diagnosed AML older than age 60 years, CRc rates with intensive chemotherapy have been between 40% and 66% with median OS of 5 to 15 months. It is noteworthy that emerging combinations such as FLAG-Ida (fludarabine, high-dose cytarabine, idarubicin, and granulocyte colony-stimulating factor) with venetoclax have demonstrated encouraging CRc rates of 61%, median EFS of 11 months, and 1-year OS of 68% in R/R AML.6 Therefore, combining E-selectin inhibitors with intensive chemotherapy and venetoclax could be a potential next step toward improving efficacy. The CRc rate of 69% with 7 + 3 with uproleselan in newly diagnosed older patients with secondary AML is encouraging when benchmarked against the 45% to 50% CRc rates noted with liposomal cytarabine-anthracycline in this population.7
Consequently the results of the placebo-controlled randomized trials of intensive chemotherapy with or without uproleselan in the first-line setting in patients age 60 years or older with 3 + 7 and in the salvage setting with MEC or FLAG-Ida are eagerly awaited (NCT03701308, NCT03616470). The ongoing trial of uproleselan with azacitidine and venetoclax (NCT04964505) is also of interest, given the lower likelihood of myelosuppression, and the potential for uproleselan to overcome resistance to venetoclax with hypomethylating agent, as are trials of uproleselan in combination with purine analogs for secondary AML (NCT04848974).8,9 Furthermore, inhibition of E-selectin may be effective when it is combined with FLT3 inhibitors to mobilize FLT3-mutated mature blasts and LSCs, because FLT3-mutated AML is often heavily dependent on E-selectin.
These findings raise intriguing questions. What is the optimal backbone for maximizing the benefit of uproleselan? Could uproleselan interfere with or blunt the mounting of a response to infectious pathogens during treatment? Which biological subgroups may benefit more, or less, from uproleselan? Given the promising outcomes with intensive chemotherapy and venetoclax, where would uproleselan-based therapies fit in the AML treatment paradigm? What are the mechanisms governing resistance to uproleselan-based approaches? Which ligands on LSCs have the maximal contribution toward the benefit noted?4 Could such approaches to destabilize the bone marrow sanctuary be harnessed to augment immunotherapy and cellular therapy approaches?
In summary, the results of this phase 1/2 study are encouraging for the field of disrupting non-immune tumor-stromal interactions that would increase the vulnerability of AML to cytotoxic therapy. We hope this approach of making the bone marrow niche less sticky pans out in phase 3 trials.
Conflict-of-interest disclosure: A.M. received research funding from Celgene. N.G.D received grants from AbbVie, Genentech, Astellas, Daiichi-Sankyo, Pfizer, Bristol Myers Squibb, Immunogen, Novimmune, and Forty Seven and had received personal fees from AbbVie, Genentech, Astellas, Daiichi-Sankyo, Pfizer, Bristol Myers Squibb, Immunogen, Jazz Pharmaceuticals, Trillium, Forty Seven, Gilead, Kite Pharma, and Novartis.